Tricresol phosphate has been around for more than a century, emerging during a period marked by accelerated growth of the chemical industry. Early on, chemists looked for alternatives to existing plasticizers, substances that could soften plastics and rubber. This compound promised improved flexibility for materials like polyvinyl chloride and cellulose nitrate, which was crucial as manufacturers shifted from brittle goods to products that could bend and stretch. Tricresol phosphate initially drew interest in Europe, where industrial laboratories demanded more versatile additives for expanding consumer markets. Over time, both military and civilian sectors sought such plasticizers for explosive formulations and electrical insulation. By the 1930s, manufacturing methods reached commercial scale, and the compound’s potential spread to Asia and North America, driven by the need for fire-resistant, easily workable materials. As decades rolled by, regulatory changes and various public health incidents forced companies and governments to rethink the widespread use of certain phosphate esters, including tricresol phosphate, putting this chemical under closer inspection and regulatory control.
Tricresol phosphate falls into the family of organic phosphates. Manufacturers often blend it with other components to give plastics increased flexibility and improved resistance to fire. Unlike some other plasticizers, this compound doesn't just mix with polymer chains; it bonds with them, raising the performance standards in demanding applications. Many industries, including aerospace and building construction, still view it as an option in cases where fire safety and durability outweigh other concerns. Different grades exist, with purity marked for technical or research use. Some producers offer stabilized versions, adding antioxidants to delay decomposition when exposed to heat or light. As regulatory scrutiny increases, buyers now expect detailed safety documentation along with shipments, including breakdowns of chemical origins and any modifications performed during the manufacturing process.
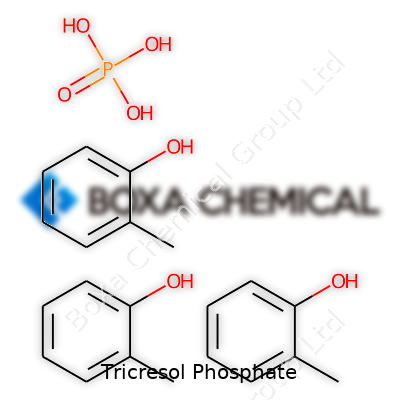
Chemically, tricresol phosphate has the structure C21H21O4P. At room temperature, the compound exists as a colorless to pale yellow, viscous liquid. Under normal laboratory conditions, its faint, aromatic odor gives a subtle hint of its phenolic origins. Its boiling point climbs above 400°C while the melting point usually stays just below 20°C. The substance dissolves easily in organic solvents such as ethanol, benzene, and toluene, but doesn't mix well with water. Chemical stability remains high under neutral and mild acidic conditions, though strong bases slowly break it down. Relative density sits just above 1.2, meaning it’s heavier than water. Heat resistance, a defining characteristic, supports its role in flame-retardant applications. These physical traits directly influence both handling procedures and downstream chemical modifications.
Spec sheets for tricresol phosphate typically call for assay levels above 99%, with moisture content below 0.1%. Most producers cap acidity—measured by free phenol—at less than 0.05% to avoid premature degradation of target polymers. Color value, assessed on a platinum-cobalt scale, must remain as low as possible to prevent discoloration of finished goods. Labels provide essential hazard symbols, batch numbers, expiry dates, and storage recommendations—usually in airtight steel drums, away from sources of ignition, and in a stable climate. Under global harmonized systems (GHS), containers must declare toxic and environmental hazard statements, alongside guidelines for accidental release and exposure management.
Manufacturing usually begins with the reaction of cresol, an aromatic alcohol, with phosphorus oxychloride (POCl3) in the presence of a catalyst. Mixing takes place in stainless steel reactors lined with corrosion-resistant materials, since both cresol and phosphorus compounds corrode ordinary metals. Temperature is managed very carefully to minimize side reactions and control byproduct formation, particularly hydrochloric acid. Once the main reaction ends, operators neutralize remaining acids using weak carbonate washes. Several rounds of liquid-liquid extraction help remove residual phenol, water, and any inorganic salts. Producers then use fractional distillation to refine the product, focusing on removing salts, colored impurities, and fractions that might compromise material properties. Packing happens under inert gas or vacuum, since the chemical oxidizes slowly in the presence of oxygen.
Tricresol phosphate responds to strong nucleophiles and bases, breaking down stepwise into cresol and orthophosphoric acid. This pathway forms the backbone of its environmental degradation. Researchers in polymer chemistry sometimes modify its aromatic rings by introducing electron-donating groups to tune solubility or improve compatibility with novel plastic compositions. In some cases, engineers use partial hydrogenation or halogenation to minimize the compound’s volatility while preserving fire resistance. The phosphate ester linkage, resistant to heat but vulnerable to enzymatic or chemical hydrolysis, often determines its fate in environmental and biological contexts. Commercial formulations sometimes anchor stabilizers or pigments to the molecule using transesterification, targeting niche markets with special demands for color stability or performance under mechanical stress.
Tricresol phosphate travels under a variety of names in trade and research circles. Chemical registries catalog it as TCP, but one can also spot references to phosphoric acid tricresyl ester, tritolyl phosphate, and tri-p-cresyl phosphate. In German literature, “Trikresylphosphat” turns up, while older English-language documents sometimes abbreviate it as TTP. Some suppliers market it under proprietary labels, pairing brand names with regulators' assigned numbers to satisfy global tracking standards. Buyers need to check the molecular formula and CAS number to make sure they're sourcing the correct compound, given the close similarity and overlapping nomenclature among several phosphate esters.
Direct contact causes irritation to skin, eyes, and mucous membranes. The compound readily absorbs through the skin. Occupational exposure has prompted strict workplace air limits in the European Union and North America. Respirators and full-length gloves figure as mandatory for anyone managing open containers. Spills call for immediate removal with absorbent material and thorough washing of exposed surfaces to prevent lingering residues. Storage demands segregation from oxidants and strong bases, with regular inspections of containers for leaks. Firefighters and first responders need to know tricresol phosphate generates toxic fumes, mainly phosphorus oxides, when burned. Workers in settings such as cable manufacturing face medical surveillance to check for early signs of toxicity. Regulatory agencies require hazard communication training and emergency response planning in workplaces using this chemical on a regular basis.
Manufacturers use tricresol phosphate for more than flexible PVC. Cable jacketing, wire enamels, adhesives, and aircraft hydraulic fluids often contain measurable amounts, benefiting from the chemical’s plasticizing and non-flammable properties. Certain inks, coatings, and lacquers bank on its balance of durability and fire resistance, with factories reporting less product loss from heat exposure. In explosives, engineers add it to propellants and munitions to slow down decomposition and improve safety margins during handling and storage. Aerospace and railway companies pick this compound for its electrical insulation ability, especially where heat resistance and stability are required. While increasing safety standards have shifted some demand to alternative substances, niche fields such as archival restoration and fine arts conservation still list tricresol phosphate among acceptable additives, usually because of its unique balance between flexibility, clarity, and resistance to light.
Since the turn of the millennium, research outfits and corporate labs have ramped up efforts to either improve or find substitutes for tricresol phosphate. Much of the new science targets improved fire retardants that cut toxicity and environmental persistence. Chemists test chemical modifications that add water solubility or reduce bioaccumulation, using bioassays, molecular modeling, and real-world environmental samples. International consortia have funded green chemistry projects that analyze breakdown products, looking for options that keep desirable properties but sidestep the risks tied to chronic exposure. Some experimental materials borrow the basic phosphate group from tricresol phosphate but graft it onto biodegradable backbones, aiming for accelerated decomposition after disposal. Patents for flexible, flame-retardant polymers often mention this compound as a legacy standard, echoed through literature even as regulatory frameworks tighten.
Much of the controversy surrounding tricresol phosphate centers on its effects in living organisms. The compound’s links to neurological damage go back to infamous poisoning outbreaks in the 1930s, when contaminated medicinal supplies caused paralysis among thousands. Lab studies confirm chronic exposure disrupts nervous system function in both rodents and humans, targeting especially the peripheral nerves. Some breakdown products build up in fatty tissues and persist for years, posing long-term health risks. Researchers regularly test for enzyme inhibition linked to neurotoxicity. Workers exposed through industrial accidents or improper protective measures report symptoms that range from tingling and numbness to permanent loss of muscle control. Even with modern safeguards, rare episodes of acute toxicity prompt recalls and industry warnings. Regulatory agencies keep updating permissible exposure limits, and new toxicity pathways emerge with advances in analytical chemistry. Environmental investigations show residues in soil and groundwater, prompting reevaluations of land disposal and recycling practices.
Tricresol phosphate faces an uncertain future as environmental and health regulations grow more restrictive. Sustainability initiatives and circular economy principles push companies to choose safer and more biodegradable chemicals. Global treaties encourage firms to phase out persistent organic pollutants, creating pressure to reformulate products and cut out high-risk additives. Startups and big chemical companies invest in alternatives, some based on renewable feedstocks, others built entirely from new chemistry paradigms. Researchers continue evaluating both the material’s benefits and its risks, knowing fire safety in critical infrastructure must balance with human and environmental health. Despite the shift, tricresol phosphate endures as a benchmark: its performance and safety history serve as yardsticks against which newcomers are measured. The chemical’s journey reflects a broader story about industrial progress, public safety, and the ongoing drive to create better, safer materials for the world’s most demanding needs.
Tricresol phosphate stands out because of its fire-resistant nature and its stable performance in harsh conditions. A lot of manufacturers turn to it when searching for a plasticizer that can also boost safety. I have seen it added to materials like vinyl and rubber, making products less likely to catch fire or melt under the pressure of high temperatures. Factories producing conveyor belts, automotive parts, or electrical cable coatings benefit from tricresol phosphate because no one wants those products failing under extreme heat.
Construction materials also depend on chemicals like this for their longevity. Think about insulation in a building, barriers resisting fire in public transport, and flooring that must not turn brittle over time. Tricresol phosphate blends into these materials and guards against serious hazards. The record backs this up: research from industrial safety journals reports that plasticizers with fire-retardant features, like tricresol phosphate, play a big part in saving lives during fires by slowing the burn time of everyday objects.
Chemical laboratories put tricresol phosphate to work as a reagent. Some lab protocols require it to test for the presence of certain metals or organic compounds. That way, it serves as a tool for quality control in medical testing and research products before they reach consumers. I recall working on a project where its presence was crucial for the accuracy of chemical analyses, especially in checking water samples for industrial runoff. The reliability it provides in lab settings often means the difference between an error and an early warning.
The same qualities that make tricresol phosphate useful also demand respect. Handling it without care leads to trouble: skin irritation, respiratory issues, and possible nervous system harm with overexposure. There have been studies by occupational health agencies showing even moderate exposure over time can affect wellbeing. Industry experience teaches to never cut corners with safety gear or ventilation around tricresol phosphate.
Stricter guidelines in Europe and North America reflect public health priorities. Labels and safe handling training come standard in companies that regularly use this compound. I have seen some businesses move to reevaluate whether they need this specific chemical or if less hazardous alternatives will do the trick. That shift matters because regulations rarely loosen when new evidence of risk surfaces.
Real progress happens as industries invest in substitutes for tricresol phosphate. Some natural-based plasticizers are coming onto the market, and early data shows they can keep fire resistance high while reducing health hazards. Scientists are trialing plant oils and phosphate blends to replace the old formulas. I am hopeful after seeing startups bring these options to scale, offering consumers safer choices and less environmental fallout.
Safer manufacturing always comes at a cost upfront, but the reduction in workplace incidents and product recalls helps balance the numbers. Swapping out longstanding chemicals is not easy—factories need to retool and staff needs retraining. The drive toward safer materials grows as companies work to boost their reputation and stay ahead of changing laws. The bottom line: knowing what goes into a product is no longer just technical knowledge; it's public trust and good business.
Tricresol phosphate doesn’t show up in everyday products most people keep at home, but you’ll spot its name around industrial settings and older machinery, especially as a plasticizer or flame retardant. I remember stepping into a storage room at a chemical plant and seeing a drum labeled “Tricresol Phosphate.” Even before I cracked a safety data sheet, just the smell hanging in the air set off warning bells. Industries trust its qualities for certain manufacturing, but the question always comes back: what does safe handling really mean for the folks working with it?
Workers sometimes tell stories about headaches, skin tingling, or an odd rash after a few hours in a poorly vented space. OSHA guidance flags tricresol phosphate as something you shouldn’t take lightly. Short-term exposure through inhalation or skin contact can lead to irritation. Studies point to nervous system effects with excessive or repeated exposure—backed by research from the National Institute for Occupational Safety and Health (NIOSH).
I’ve met technicians whose gloves failed during cleanup tasks. Even with quick rinsing, some ended up with irritated wrists and itching that stuck for days. It’s one of those chemicals that seem harmless at a glance, but if you let your guard down, it can land you in the occupational health office.
Always respecting the material makes a difference. I recall once monitoring a training session at a facility in the Midwest; workers used goggles, chemical-resistant gloves, and long sleeves, no exceptions. Plenty of breaks for handwashing, automated ventilation systems running strong, and safety showers within reach. These aren’t just box-ticking exercises. Incidents often pop up when folks skip steps on a rushed day or the personal protective equipment (PPE) looks “good enough” but isn’t rated for organic chemicals.
Safety data sheets matter. In my experience, it helps when employers post quick-reference information directly above chemical storage—easy to find in an emergency. Clear labeling and regular safety drills can keep everyone a bit sharper. Pair that with regular airflow checks and prompt cleanup of spills, since organic phosphates like this can stick to surfaces.
Research still chases questions about tricresol phosphate and cancer or chronic health effects. Some rodent studies hint at longer-term nervous system problems, but these results haven’t translated cleanly to human settings. That kind of uncertainty gives me pause, since it means strict caution keeps you out of trouble in the absence of hard answers.
Trusting your nose or eyes only stretches so far. Air monitoring, good PPE, regular checkups—these basics keep workers healthy. Supervisors who set clear rules make a real difference. Holding short morning safety huddles or anonymous suggestion boxes about air quality helps, too. Nobody works well while worried about invisible hazards on their skin or floating through the air.
Instead of hoping for zero incidents, hiring teams who care about up-to-date training and prompt reporting of symptoms supports a safer work environment. Modern plants using substitutes or enclosed systems can further shield workers from risks, showing how innovation and care tie together. Even as tech advances, experienced hands and sharp eyes give the best protection—no shortcut beats knowledge and respect for the chemical in the drum.
Tricresol phosphate carries a reputation for being more than just a specialty chemical. Folks who have spent time in manufacturing, especially around old-school plastics or tough industrial coatings, have seen this substance put through its paces. Many know it for its use in the plastics sector, where flexibility and endurance count for more than anything. PVC production relies on plasticizers, and tricresol phosphate stands out for making products less fragile and more workable. Wiring insulation, conveyor belts, and even artificial leather owe their feel and durability partly to TCP’s flexibility boost. Working with these materials, I’ve noticed firsthand how wires bend smoothly without cracking—a direct benefit of quality plasticizers like tricresol phosphate.
People value fire prevention, and tricresol phosphate answers this demand. Its chemical structure adds flame-retardant properties to various products. Furniture foam, cables, and textiles often include TCP because it hinders ignition and slows burning. Reliable safety matters to families and builders, especially in dense cities where fires can spread quickly. I’ve seen fire-resistant foam cushions tested, and products containing tricresol phosphate consistently buy precious extra minutes for escape or firefighting efforts. Factories and builders favor these materials not just for compliance, but for genuine risk reduction.
Besides plastic and fire safety, TCP enters the spotlight in fluid engineering. Engineers searching for lubricant additives that withstand high stress and temperatures long ago settled on tricresol phosphate in select applications. It strengthens oils so that moving metal parts last longer without seizing up. Industrial turbines and aircraft hydraulic systems draw from this technology. On the shop floor, extended downtime from failed hydraulics can grind work to a halt—no one wants that. Oils bolstered with additives like TCP keep the operation running smoother for longer intervals, lowering repair bills and improving confidence in hard-working machines.
Despite these helpful uses, people in labs and manufacturing plants keep a careful eye on handling and exposure. Evidence has pointed to toxicity risks if not managed properly. Chronic exposure can hurt the nervous system, which sets off alarms for employee health. Good ventilation, thorough training, and updated handling rules, like those set by OSHA and EU REACH, play a major part in protecting workers. I recall hearing about safety upgrades at a plant, where older practices gave way to sealed processes and regular health checks—simple steps, but with a measurable impact on worker well-being.
Public attention on chemical safety keeps growing. Researchers and product developers investigate greener, less toxic alternatives to tricresol phosphate for sensitive uses, especially items that come into everyday contact with children or food. Independent groups now publish comparative results and help guide companies toward replacements, without losing out on quality or performance. Industry adoption isn’t always fast, but consumer demand and updated regulations push companies to modernize recipes and safety protocols.
Tricresol phosphate’s primary jobs—plastics, fire resistance, and lubricants—support major parts of daily life. Strong oversight, smart engineering choices, and open-mindedness toward new options keep the risks lower and the benefits stronger.
Tricresol phosphate carries the chemical formula C21H21O4P. Chemistry sometimes hides behind complicated terms, but this one deserves a straightforward look. Each molecule forms from three cresol units attached to a single phosphate group. That structure puts it among the family of organophosphates, but tricresol phosphate stands apart because of its specific cresol backbone. This composition supports several specialized roles in industry, but it also brings unique risks that shouldn’t get brushed aside.
Factories do not use tricresol phosphate by accident. Its main calling comes as a plasticizer—meaning it makes plastics such as cellulose acetate less brittle and more workable. No one enjoys rigid, cracking plastics in devices or equipment. That need alone put tricresol phosphate to work in the twentieth century, shaping everything from insulation coatings to films and some lacquer finishes.
Its utility stretches beyond softening plastic; it shows up occasionally in hydraulic fluids and as a flame retardant in some electrical applications. Three cresol rings bonded to a phosphate core result in a stable, oil-like liquid, resistant to heat and chemical breakdown. Durability matters especially in manufacturing settings where extreme temperatures or corrosive chemicals turn up every day. Standard plasticizers might break down, but tricresol phosphate hangs in longer under stress.
We can’t talk about tricresol phosphate without addressing safety. Like other organophosphates, it doesn't always play nice with human biology. Long-term or high exposure has the potential to affect the nervous system and liver. This isn’t just lab data; chemical plants over the years reported cases of nerve damage linked to organophosphate exposure, tricresol phosphate included.
The big-picture lesson: factories and labs treat tricresol phosphate with respect for a good reason. That means ventilation, gloves, and strict workplace controls form everyday routines wherever the chemical gets used. I’ve seen crews at older facilities spend more time on safety checks around organophosphates than almost any other organic liquid. Regulations set by bodies like OSHA or the EU’s REACH reflect lived experience. These rules didn’t appear out of thin air—they grew from incidents, medical monitoring, and the concrete effects of overlooking proper safeguards.
Industry keeps searching for replacements. Less toxic plasticizers based on phthalates, adipates, or even bio-based solutions push into markets previously reserved for tricresol phosphate. The transition isn’t always seamless. Not every alternative can offer the same mix of heat resistance and flexibility. Still, new research nudges companies to swap out old chemicals once better solutions hit the market. The push for “greener” chemistry gains more supporters each year, powered by tighter environmental rules and informed employee concerns.
For anyone learning about or working with industrial chemicals, knowing the formula—C21H21O4P—gives more than trivia value. That knowledge unlocks an understanding of both the molecule’s power and its hazards. Past events and the ongoing hunt for safer chemicals shape the way we handle such compounds in real-world workplaces, showing why chemical literacy remains vital in industry and public health.
Plenty of folks handle chemicals every day, but only a few pause to think about what’s actually around them. Tricresol Phosphate doesn’t make headlines, yet storing it correctly keeps people, equipment, and the environment out of harm’s way. I’ve seen what sloppy storage can do—labels peeking off, stains on the shelf, people coughing in the next room. Those might seem small at first, but risks add up quick.
This compound carries more weight than a label lets on. It works as a flame retardant and plasticizer, and scientists use it in labs all over. But that same utility brings real-life downsides. World Health Organization data lists Tricresol Phosphate as a chemical with toxic potential if not handled with care. Fumes, skin contact, and spills shouldn’t become part of anyone’s routine.
To steer clear of problems, Tricresol Phosphate belongs in a spot out of reach from kids and pets. A locked cabinet works well, away from daylight and far from any flames or sources of heat. Some labs cut corners and stack bottles next to windows or radiators. Sunlight and heat can break down chemicals, letting fumes escape or changing the compound in ways you don’t want to discover down the road. I learned to treat every bottle the same, fresh or half-empty: keep it cool, dry, sealed tight, and away from curious hands.
Mixing chemicals turns a safe room into a hazard zone. Tricresol Phosphate doesn’t get along with oxidizers, acids, or open bottles of other industrial compounds. In my experience, one poorly labeled shelf made for a hard afternoon—spills blend, things bubble up, and you’re suddenly evacuating a room. Store Tricresol Phosphate away from other reactive chemicals. Using a secondary containment tray catches leaks before they become disasters. Sticky residue on a shelf tells you something’s already gone wrong.
Safety matters most. No one wants a trip to the emergency room because of skin contact or fumes. I’ve always put on gloves and goggles, even if I’m just checking a label or shifting a bottle to the back. The CDC and Occupational Safety and Health Administration both stress the basics: gloves, protective eyewear, a lab coat—or at minimum, sturdy clothing. Ventilated rooms or chemical fume hoods cut down on accidental exposure.
Handwritten notes fade. Every container should show what’s inside, when it arrived, and who last checked it. I use bold labels, and update inventory lists every month. This way, nothing surprises you after a long weekend or shift change.
Everyone wants to get rid of leftovers one day. Tricresol Phosphate demands proper disposal—corrosive chemicals and city trash bins don’t mix. Local hazardous waste laws give the best guidance. Emergency plans deserve attention just as much. Spills do happen, so have absorbent materials, neutralizing agents, and clear instructions posted for your whole team. Regular training makes these steps second nature, stopping panic before it starts.
Storing Tricresol Phosphate might sound like a chore, but getting it right keeps spaces safer for everyone. Good habits build trust between co-workers and keep surprises to a minimum. With straightforward routines, most chemical risks shrink back to size.
| Names | |
| Preferred IUPAC name | Tris(2-methylphenyl) phosphate |
| Other names |
Tricresyl Phosphate TCP Phosphoric acid, tris(methylphenyl) ester Tri-cresyl phosphate Trixylenyl phosphate |
| Pronunciation | /traɪˈkriːsɒl ˈfɒsfeɪt/ |
| Identifiers | |
| CAS Number | 1330-78-5 |
| Beilstein Reference | 516104 |
| ChEBI | CHEBI:45055 |
| ChEMBL | CHEMBL1486 |
| ChemSpider | 54696 |
| DrugBank | DB11106 |
| ECHA InfoCard | 050000013189 |
| EC Number | 204-112-2 |
| Gmelin Reference | 8983 |
| KEGG | C17712 |
| MeSH | D014267 |
| PubChem CID | 6626 |
| RTECS number | TF9625000 |
| UNII | 43M16S235D |
| UN number | UN2574 |
| Properties | |
| Chemical formula | C21H21O4P |
| Molar mass | 368.37 g/mol |
| Appearance | Colorless crystalline solid |
| Odor | odorless |
| Density | 1.215 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 3.86 |
| Vapor pressure | <0.01 mmHg (@ 20°C) |
| Acidity (pKa) | 1.15 |
| Basicity (pKb) | 13.2 |
| Magnetic susceptibility (χ) | -76.0e-6 cgs |
| Refractive index (nD) | 1.553 |
| Viscosity | 30-40 cP (at 25°C) |
| Dipole moment | 2.8 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 262.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -833.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7453 kJ/mol |
| Pharmacology | |
| ATC code | D08AX01 |
| Hazards | |
| GHS labelling | GHS02,GHS07,GHS08 |
| Pictograms | GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: - |
| Flash point | 154°C |
| Autoignition temperature | 410°C |
| Lethal dose or concentration | LDLo (oral, human): 214 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3,650 mg/kg (oral, rat) |
| NIOSH | TF1575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Tricresol Phosphate is "0.1 mg/m³ (as TWA)". |
| REL (Recommended) | REL (Recommended): 0.05 mg/m³ |
| Related compounds | |
| Related compounds |
Cresols Phosphoric acid Triphenyl phosphate Tricresyl phosphate |