Tri-O-Cresyl Phosphate doesn’t show up on the evening news, but its story goes back nearly a century. Early chemists searching for new ways to make plastics more flexible stumbled upon organophosphates like this one in the years before World War II. Large manufacturers, eager to outpace rivals in producing better paints and resins, threw serious resources at phthalates and others, but organophosphates grabbed the chemistry world’s attention thanks to their odd combination of traits. By the forties and fifties, industrial adoption spread across North America and Europe as companies realized this compound could make airplane parts and cables tough yet flexible. My time in industry showed me that materials people keep using over decades almost always leave complicated legacies, both good and bad.
Tri-O-Cresyl Phosphate presents itself as a slightly viscous, colorless to pale yellow liquid in its purest traded forms. You’ll see it in steel tanks at factories, but also—if you dig into the ingredients list of some older electrical insulation—inside the wiring of legacy subway systems and aircraft. The stuff finds daily use mainly as a plasticizer. Wherever flexibility in a vinyl or polymer is needed, the chemical often plays a quiet supporting role. Demand remains steady due to its superior performance in resisting heat and plastic fatigue.
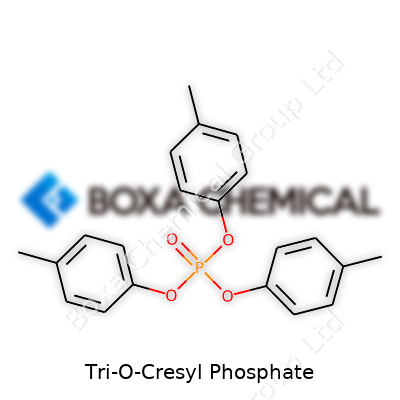
Chemically, Tri-O-Cresyl Phosphate consists of three cresol groups linked by a phosphate core. Its molecular weight is about 368 g/mol. Dipping a thermometer in, you’ll hit its boiling point at roughly 410°C, well above kitchen temperatures, and the liquid refuses to freeze until below -30°C. Solubility in water stays low, but it mixes readily with other organic solvents such as benzene or ether. This versatility prompts manufacturers to reach for it repeatedly. The compound's flame retardant qualities stem from its phosphate; exposure to open flame causes it to char and absorb heat, protecting nearby material rather than feeding the fire.
A factory shipment comes labeled according to a strict international system. You spot hazard warnings for organophosphate toxicity, UN number 2574, and notes about safe handling. Manufacturers typically specify content purity (over 99%), maximum allowable levels of isomeric impurities, and precise viscosity ratings at 25°C. Engineers need to check density when blending it into a new batch—look for a value just above 1.2 g/cm³. In many labs I’ve worked alongside, labeling gets double-checked by safety teams, as incorrect or missing hazard icons can have life-changing consequences.
Large chemical plants synthesize Tri-O-Cresyl Phosphate by reacting cresols with phosphorus oxychloride, using a catalyst and strict temperature controls. This treat-and-watch process releases hydrogen chloride gas, so exhaust management becomes a critical step—acidic steam requires cubic meters of scrubber and vent space. I’ve chatted with older plant operators who recall early, less regulated days when the fumes made their eyes water. Today’s processes focus on recycling phosphate and minimizing releases, but the core reaction—esterifying cresols—hasn’t changed in decades.
This molecule resists acid hydrolysis but can break down with hot, concentrated alkali, splitting into cresol and orthophosphoric acid. Careful chemical engineers modify the compound by swapping meta, para, or ortho positions of the cresol arms, influencing properties like volatility and resistance to decomposition. Improvements in the purification process, using fractional distillation, reduce hazardous byproducts like ortho-cresyl isomers. These details matter on the shop floor since even a small increase in reactive impurities can spoil whole factory runs of polyvinyl chloride or insulation sheathing.
You won’t find Tri-O-Cresyl Phosphate just under one name. Around the globe, it answers to brand names like TOCP, tricresyl phosphate, and phosphate de tricrésyle. Labels in factories might use abbreviations—TCP in the States, or sometimes just “trio-cresyl” on street-level shipping forms. Tracking down information in data sheets or academic work requires running through these aliases, as regulatory filings and chemical suppliers rarely standardize names across borders.
Working with Tri-O-Cresyl Phosphate draws a sharp line between today’s labs and those of the 1920s. Plant staff receive regular training on organophosphate risk management. Gloves, splash goggles, and full-face respirators remain the norm anywhere fumes or mist might rise. Proper ventilation and fume extraction systems offer a line of defense—failures lead to quick headaches, longer exposures can trigger nausea or nerve pain. A lab incident I witnessed—where a careless technician splashed solvent on bare arms—underscored how little margin for error the compound leaves. Global standards like REACH in the EU and the EPA’s inventory in the US track commercial shipments, requiring Material Safety Data Sheets at each transfer. Modern production plants schedule routine air monitoring for airborne levels, and facilities install chemical spill kits throughout the workflow.
Polyvinyl chloride reigns supreme as the main beneficiary of this plasticizer’s abilities. Power cables flexing through tunnels rely on it. Early aircraft designers looked to TOCP for flame resistance, a blessing in engine nacelles or cockpit insulation. Railcar manufacturers depended on it to protect wiring from oil and moisture. In hydraulic fluids, the lubricant’s non-flammability kept critical systems running in hot environments without catching fire. Despite a shrinking footprint in consumer goods, the compound still turns up in some legacy industrial coatings and paints. You rarely see it marketed to the public, but its invisible hand steadies more parts of the infrastructure than many people realize.
Since the 1970s, a wave of research teams worldwide investigated substitutes and process improvements, prompted by rising concerns about chronic toxicity. Biochemists dissected breakdown products, flagging certain isomers as particular risks to living cells. In university projects I reviewed, enzyme-based sensors and analytical techniques grew more sophisticated, letting teams trace metabolites to ever lower levels in wastewater and workplace environments. Meanwhile, commercial labs tinkered with new plasticizer blends, chasing better fire safety without the health compromises. Some biotech startups claim enzyme catalysts will eventually create safer, cleaner alternatives, but scaling up these lab wins to a production plant keeps proving harder than it seems.
The health hazards linked to Tri-O-Cresyl Phosphate reach back to infamous poisoning cases in Prohibition-era America, where contaminated 'medicinal' drinks left thousands paralyzed. Neurologists traced the damage to specific impurities—especially ortho isomers. Animal studies, cell cultures, and epidemiological surveys since the 1950s consistently find risk at higher exposure levels. The science supports a clear line: chronic or cumulative contact with unprotected skin, or long-term inhalation, can trigger nerve damage and disrupt enzymes crucial for the nervous system. Strict handling rules and technical modifications to reduce the most toxic isomers have cut the rate of incidents, but regulators and activists remain wary. Some large producers switched supply entirely to safer compounds after failed litigation or worker illness.
Tighter environmental laws and the ongoing search for greener chemistry place Tri-O-Cresyl Phosphate at a crossroads. Many industries once dependent on it now look for drop-in replacements, especially in Europe and North America where standards only get tougher. Yet, outright bans spark pushback from manufacturers still betting on familiar processes or facing high costs to retool. I’ve sat in on lively trade conferences where engineers and regulators lock horns over how much risk is too much—progress looks incremental, moving inch by inch toward safer, renewable solutions. Smart investment favors next-generation plasticizers that hold up under heat and outlast rivals without the legacy of nerve damage. There’s hope in the pipeline, but for now, the story of Tri-O-Cresyl Phosphate reflects the messiness of real-world chemistry, where every innovation leaves a mark, for better or worse.
Tri-O-Cresyl Phosphate, which a lot of people in industry call TOCP, is a chemical that’s been around for over a century. It belongs to a family of organophosphates and shows up mainly as a colorless to pale yellow liquid with a faint odor. Factories and labs don’t pick this compound for no reason — it’s got real-world uses in helping products work better and last longer. But like many chemicals, TOCP carries baggage that deserves attention.
TOCP is widely used as an additive in lubricants for engines and turbines. Imagine the kind of power found in aircraft jet engines, where both heat and pressure punish moving parts round the clock. TOCP works as an anti-wear agent here, helping metals move smoothly against each other. Some people in aviation say the right additives make the difference between an incident-free flight and unexpected repairs on the ground.
Companies use TOCP as a plasticizer. That means it gives plastics more flexibility and resilience. If you own old electrical cords, vinyl flooring, or synthetic leather, there’s a chance you’ve handled something made with this chemical. It helps soften rigid plastics so they won’t crack easily or become brittle with use. Factories also tap into TOCP's fire-resistant properties. In products with strict safety codes, adding this chemical reduces how quickly flames can spread, buying people and firefighters valuable seconds.
Industrial machines don’t get breaks. When I toured a hydraulic systems plant, the managers pointed out the fluids that keep factory robots moving smoothly. TOCP shows up in some specialty hydraulic fluids and greases. In these places, reliable performance beats almost every other priority. The difference between a routine workday and thousands lost in downtime often hinges on such choices. Choosing additives that limit corrosion or breakdown can extend equipment life and prevent environmental leaks.
With all its practical uses, TOCP brings serious risks if mismanaged. Stories from the early 20th century spoke of workers exposed to TOCP who later suffered nerve damage. Some countries quickly put limits in place. We know today exposure through skin contact or inhalation can cause neurological problems, sometimes years after the fact. The lesson here is simple: what makes machines run smoothly can still be hazardous if rules aren’t followed.
Modern industry pays close attention to worker health. Many companies now look for replacements or keep TOCP at the lowest possible levels. Factories where this chemical turns up follow strict protocols — gloves, air filters, and regular medical checks. In the past, not enough was known. Today, more eyes are on both human safety and what seeps out into the ground or water. Research teams look into new molecules that match TOCP’s perks with fewer dangers.
Every chemical like TOCP sits at a crossroads between usefulness and safety. My time in manufacturing showed me just how tightly safety and efficiency are intertwined. The chemists and engineers who decide whether to use TOCP face an ongoing challenge: making things last, while never letting yesterday’s mistakes repeat. Choosing safer work practices and pushing for better options both matter. As everyday folks, staying informed helps keep industries and regulators accountable to people, not just profit margins.
Tri-O-Cresyl Phosphate, often called TOCP, crops up in everything from plasticizers for PVC to hydraulic fluids and even some flame retardants. Not many realize that it once found its way into products like aviation lubricants and industrial machinery. I remember hearing stories about workers in the early 20th century, some losing their motor control after handling certain fluids containing this chemical. Today, the stakes are just as real, but many are unfamiliar with the nature of the risk.
TOCP exposure mostly happens through inhalation or skin contact. Toxicologists have zeroed in on its link to a nervous system problem called organophosphorus-induced delayed neuropathy, which sounds clinical, but the reality is tough: tingling in the legs, muscle weakness, and in severe cases, actual paralysis. Back in 1930, a contaminated batch of ginger extract laced with TOCP left thousands in the U.S. with nervous system damage—a disaster that pushed researchers to look much closer at organophosphates like this one.
The Centers for Disease Control and Prevention classifies TOCP as hazardous. Chronic exposure, even at low doses, can damage nerves for good. I once met a chemist who worked next to a drum of TOCP for years without proper gloves. By the time he realized what was happening, his hands would shake when he tried to hold a pencil. These stories don’t often make the news, but they stick with the communities where manufacturing and maintenance jobs involve these chemicals.
The Occupational Safety and Health Administration (OSHA) sets rules for safe handling, like using proper gloves, goggles, and ventilation. Following these isn’t just a legal requirement, it makes good sense. Simple habits—good ventilation, washing up after handling chemicals, not eating in contaminated areas—go a long way. Companies that keep up regular training and make sure workers know what they’re handling see far fewer health issues tied to TOCP.
One trouble spot lies in old machinery and older buildings. Without careful labeling or up-to-date material safety data sheets, people can run into TOCP without knowing. I once spoke to a retired aircraft mechanic who’d spent decades with little more than a rag for protection. Only later did he learn about the risks from safety briefings, after his hands started tingling. That lack of information happens too often, especially in smaller shops or places where regulations aren’t enforced as tightly.
Some companies have switched to safer alternatives, using newer plasticizers and lubricants that don’t harm nerves. Research keeps moving forward, but TOCP still surfaces in imported goods and second-hand equipment. Disposal and recycling also present trouble; improper dumping can slip this nerve toxin into groundwater and soil, threatening whole communities.
It takes more than law to make a difference—it needs a culture where people share what they know and question what’s not labeled or explained. If a chemical causes harm across generations, there’s a strong case for replacing it or safeguarding workers with everything science and experience can offer. At the end of the day, no job is worth sacrificing health to a hidden hazard.
Tri-o-cresyl phosphate, known in the chemistry world as TOCP, stands out as one of those industrial chemicals with a story. Over the years, it’s found its way into plenty of manufacturing routines. I’ve come across GC-MS reports that pick up TOCP in plastics and lubricants, especially where you need to balance performance and safety. Its mix of three cresol molecules bonded to phosphate gives it some interesting character, both in the lab and beyond.
Bring a sample of TOCP into the light, and you’ll spot a colorless to pale yellow oily liquid. It doesn’t grab attention with any strong smell, which can lull you into underestimating its presence. Try pouring it, and you’ll find it leans heavy and doesn’t evaporate quickly. Its boiling point hangs up around 410°C (770°F), so TOCP can handle plenty of heat before it starts turning to vapor. Its density—just below water, around 1.18 g/cm³—lets it sink if spilled into most liquids without much drama.
It won’t dissolve in water easily, something I’ve seen frustrate cleanup crews after an accidental splash. Send it into most organic solvents, though—like ethanol or benzene—and TOCP mingles in. That makes it easy to blend into oils, which is the reason folks stick it into plasticizers and flame retardants.
Ask any industrial chemist, and they’ll tell you TOCP brings a steady hand in chemical mixtures. It stands up to light and moderate heat without falling apart. In my time working with polymer additives, TOCP never broke down during normal process conditions. This stability keeps equipment humming and products consistent.
Its structure—three cresyl rings linked to a phosphate—gives it an edge in fending off flames. Those phosphate groups disrupt combustion, which is why TOCP shows up in flame-retardant formulas. That same sturdy structure means it doesn’t react fast with acids or bases at room temperature. Start pushing temperatures or add strong oxidizers, and you’ll see decomposition; no one wants those fumes drifting through a work site.
The dark side of TOCP casts a shadow over all its industrial uses. History remembers the ‘Ginger Jake’ tragedy in the early 20th century—a mass poisoning from contaminated prohibition-era liquor. TOCP slipped past the senses: tasteless, odorless, and nearly invisible, but toxic to nerves. Exposure can leave damage that lasts years, if not for life. This lesson turned worker safety and strict handling rules into non-negotiables across much of the world.
I’ve seen how modern labs handle any TOCP spill—gloves, masks, ventilation, and immediate isolation. The Occupational Safety and Health Administration (OSHA) lays out exposure limits for a reason. Any work involving TOCP now comes with training, eye-wash stations, and testing protocols. These measures keep accidents rare, but complacency always carries a high price.
The long shelf-life and chemical toughness of TOCP keep it relevant, but it pressures industries to research safer alternatives. Academic groups and manufacturers keep searching for plasticizers and retardants with less risk to nerves and the environment. In my experience, swapping out tried-and-true chemicals calls for collaboration—scientists, safety officers, and policy-makers working together. Sticking to strict monitoring and investing in research can prevent old mistakes from repeating themselves. Today, getting familiar with the properties of TOCP isn’t just for chemists—it helps keep communities, workers, and the environment safer.
Tri-O-Cresyl Phosphate (TOCP) does a lot of heavy lifting in industry, especially as a plasticizer and flame retardant. The stuff can prove hazardous, though, and not just in far-fetched accident scenarios. Real people have gotten sick by breathing fumes or touching it without proper protection. In the 1930s, a contaminated batch of cooking oil caused paralysis in thousands. That tragedy sticks in the minds of chemical handlers for a good reason—it reminds everyone what sloppy storage and careless use can lead to.
Keeping TOCP locked up changes more than guidelines or regulatory compliance sheets. It’s about making sure workers clock out healthy. This chemical comes as a viscous liquid, and it shouldn't end up in the wrong place. The best approach involves a dedicated, well-ventilated storeroom. Only folks trained to recognize and manage risks should have keys. Drum lids and containers must seal tightly, and it pays to keep them off the ground on pallets. That simple step guards against leaks if containers rupture or overflow.
No one wants to stand next to a chemical footprint waiting for someone else to mop it. Absorbent materials should be on hand, easy to grab if a spill happens. Every container needs a clear label—a real label, not just a marker. That small effort prevents confusion, especially during shift changes or emergencies.
My first apprenticeship in a chemical plant taught me what gloves and goggles are really for. I always thought safety gear felt uncomfortable until a splash landed near my eye and reminded me why old hands never skip PPE. Nitrile gloves keep TOCP away from the skin, and splash-proof goggles prevent accidents from turning into hospital visits. Full coveralls and sturdy shoes take a little getting used to but pay off by stopping contact with clothes or skin.
Handling TOCP goes beyond equipment—ventilation matters. A fan by itself doesn’t cut it. Mechanical exhaust systems in the workspace lower concentrations of airborne chemicals, which becomes vital if you spend hours in a storeroom or mixing area. Breathing protection, such as an approved respirator, matters most during transfers or spills.
Training beats posting a list of safety rules. I learned more from hands-on safety drills than from skimming an instruction manual. Workers should walk through spill responses and know the quickest exit routes. Cleaning crews need to know what to do with waste, from rags to contaminated soil—never toss these in normal trash bins.
Emergency eyewash stations should never be out of reach. A clear sign marks them yes, but regular inspection counts for more. During inspections in my last job, a blocked eyewash delivered a wake-up call. Since then, surprise drills became our best habit.
Transparency with the team builds safety culture. Anyone who hears about a storage issue or sees damage must feel comfortable reporting it, not hiding it. Supervisors set that tone daily—not by lecturing, but by example. Keeping inventory logs, reporting near-misses, and rotating stock prevent surprises in the storeroom.
No storage or handling system guarantees zero accidents, but consistent care, learning from past errors, and honest oversight go a long way. People’s health stands on the line with every decision about TOCP. If you treat it as routine, regret follows. Treat it seriously, and risks shrink fast.
Tri-O-Cresyl Phosphate, better known as TOCP, has earned strong opinions because of its links to health problems. It’s found in jet engine oils, some hydraulic fluids, and older electrical equipment. Some manufacturers use it because it resists heat and doesn’t burn easily. The history of TOCP includes a painful lesson—an outbreak in the 1930s, when people suffered nerve damage after exposure through contaminated ‘Ginger Jake’ medicine. Anyone who handles TOCP looks back at that story and remembers what’s at stake.
For workers, a safe job site depends on clear rules. The Occupational Safety and Health Administration (OSHA) in the U.S. sets limits for dangerous chemicals, including TOCP. OSHA’s permissible exposure limit (PEL) for TOCP stands at 0.1 mg per cubic meter of air (averaged over an 8-hour shift). European agencies like ECHA also keep strict guidelines, demanding regular monitoring and detailed labeling. There’s no guesswork—labels read “May cause damage to organs through prolonged or repeated exposure.”
Personal protective equipment turns theory into practice. Lab workers dealing with TOCP pull on gloves made of materials like nitrile or butyl, lab coats, and goggles. I’ve watched coworkers double-check their respirators, especially in tight spaces with weak air circulation. Engineering controls count, too—fume hoods or full ventilation systems now show up in plants or labs where exposure might creep up. Regular air sampling tells the safety staff whether their controls hold the line.
If someone spills TOCP, the worst move is grabbing paper towels and hoping for the best. Clean-up crews suit up, block off the area, and use absorbents that lock away chemicals. Waste lands in sealed containers tagged for hazardous materials pickup, not the regular trash. Training matters most—I’ve attended sessions where we drilled how to spot early symptoms of exposure, such as tingling hands or clumsy fingers. Timely reporting can save a worker’s nerves, literally.
Some countries push harder than others. Japan banned TOCP from most consumer products, and the European Union adds layers of regulation under REACH. The U.S. EPA lists TOCP under its Toxic Substances Control Act. Gaps pop up in places with weaker infrastructure, where old equipment still holds onto toxic oils. Surplus equipment, especially from military or industrial sources, often gets shipped overseas. This backdoor flow brings risks where oversight trails behind.
It’s easy to say “follow the rules,” but pressure to cut corners runs high in fast-moving industries. Stronger auditing and whistleblower protections support workers who speak up. Newer, less toxic additives can replace TOCP in some uses, though switching costs time and money. Sharing information across borders helps—if one company figures out a safer way, others should learn, not stumble through the same old risks. Technology keeps pushing the conversation, with sensors that offer real-time detection in workspaces. Early warnings give workers a better shot at staying safe, and that’s a lesson no one wants to relearn the hard way.
| Names | |
| Preferred IUPAC name | Tris(2-methylphenyl) phosphate |
| Other names |
TOCP Tricresyl phosphate Phosphoric acid, tricresyl ester Tris(o-cresyl) phosphate Tri-o-tolyl phosphate Cresyl phosphate |
| Pronunciation | /traɪ.oʊˈkriːsəl ˈfɒsfeɪt/ |
| Identifiers | |
| CAS Number | 78-30-8 |
| Beilstein Reference | 4142529 |
| ChEBI | CHEBI:35050 |
| ChEMBL | CHEMBL14222 |
| ChemSpider | 20203 |
| DrugBank | DB14005 |
| ECHA InfoCard | 01d20b0c-cf7e-4b47-bf32-793e7de9b9d6 |
| EC Number | 204-112-2 |
| Gmelin Reference | 65541 |
| KEGG | C07298 |
| MeSH | D014273 |
| PubChem CID | 6626 |
| RTECS number | GL7875000 |
| UNII | FMB1XS5606 |
| UN number | UN2574 |
| CompTox Dashboard (EPA) | DTXSID5020722 |
| Properties | |
| Chemical formula | C21H21O4P |
| Molar mass | 368.39 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 0.00006 mmHg (25°C) |
| Acidity (pKa) | 1.39 |
| Basicity (pKb) | 6.4 |
| Magnetic susceptibility (χ) | -62.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.551–1.556 |
| Viscosity | 3.45 cP at 25°C |
| Dipole moment | 2.91 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 347.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1247 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7213 kJ/mol |
| Pharmacology | |
| ATC code | D04AA09 |
| Hazards | |
| Main hazards | Toxic if swallowed. Causes damage to organs through prolonged or repeated exposure. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H373 |
| Precautionary statements | P210, P261, P280, P301+P310, P305+P351+P338, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-2-0 Health:2 Flammability:2 Instability:0 |
| Flash point | 225°C (closed cup) |
| Autoignition temperature | 410°C (770°F) |
| Lethal dose or concentration | LD50 oral rat 367 mg/kg |
| LD50 (median dose) | 19 mg/kg (oral, rat) |
| NIOSH | TT2975000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Tri-O-Cresyl Phosphate: 0.1 mg/m³ |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | 40 mg/m3 |
| Related compounds | |
| Related compounds |
Diisopropyl phosphite Diphenyl phosphite |