Chemists in the early twentieth century recognized the unique properties of benzoquinone derivatives, leading to the first reports of tetramethyl-1,4-benzoquinone synthesis around the 1920s. Early research was curiosity-driven, centered on exploring new quinone compounds for potential industrial and research uses. As organic chemistry advanced, scientists started to understand how the methylation pattern of the aromatic ring changed not just the stability of the molecule, but also its reactivity and functional versatility. The compound’s journey from laboratory curiosity to essential chemical tool reflects how persistent investigation and technical progress unlock hidden value from once-minor substances.
Tetramethyl-1,4-benzoquinone appeals to both synthetic chemists and industry alike, simply because it does what few compounds can. Known for acting as a selective oxidant, this quinone provides chemists flexibility for designing cleaner reaction pathways. Its structure, featuring four methyl groups on a standard 1,4-benzoquinone framework, makes it stable but still reactive enough to take part in a wide range of transformations. In my projects, choosing this quinone has been a reliable way to push certain delicate reactions across the finish line. Laboratories around the world keep it on hand for both routine syntheses and as a benchmark substrate when developing new reaction methodologies.
Typically appearing as a bright yellow crystalline solid, tetramethyl-1,4-benzoquinone draws attention with its recognizable aroma and sharp, persistent odor. Its melting point lies close to 160°C, and thanks to the methyl groups, it dissolves in many organic solvents. The structure boosts both lipophilicity and chemical resilience, making it less prone to hydrolysis in ambient conditions compared to some of its less substituted cousins. Electrochemical properties set it apart as an attractive electron acceptor, which gets put to work in both organic and materials science research. Personally, it has offered an interesting case study when demonstrating how slight changes in structure can dramatically alter both physical handling and reactivity.
Most suppliers specify a purity of at least 98% to ensure reliability in sensitive synthetic applications. Production batches always come with a material safety data sheet, highlighting storage needs away from direct sunlight and moisture, plus clear hazard identification for proper lab practice. Precise batch records and clear labeling support traceability and minimize confusion in busy laboratory settings. Chemical suppliers also include the molecular formula (C10H12O2), molecular weight (164.20 g/mol), along with spectroscopic data like NMR and infrared references to verify identity. This transparency has proven essential in my own experience, ensuring each shipment matches previous results and eliminates unnecessary troubleshooting or rework.
Most reliable syntheses start with durene (1,2,4,5-tetramethylbenzene) as the raw material, oxidizing it with agents such as chromic acid or potassium dichromate in acidic medium. The process delivers the quinone as a crude product, which purification—typically using recrystallization—brings to a high standard. The choice of oxidant impacts both yield and environmental footprint, and with recent attention on sustainability, more labs are switching toward greener oxidants like hydrogen peroxide. On small scale, careful temperature control during oxidation keeps side products in check, a lesson I've had reinforced during more than one bench-top trial in academic labs.
Tetramethyl-1,4-benzoquinone interacts with nucleophiles and participates in both addition and reduction reactions. Chemists have used it as a mediator for electron transfer in organic synthesis, as well as a mild oxidant for transforming alcohols or sulfides. It can act as a Michael acceptor for select conjugate addition reactions, offering a chance for further functionalization. Reactions with organometallics or catalysis under photochemical conditions open doors to even more transformation types. The organic electronics sector explores its redox chemistry as well, aiming for new applications in battery and sensor technology, which points to how much innovation flows from a core reagent like this.
Across the chemical literature and commercial catalogs, tetramethyl-1,4-benzoquinone crops up under several names—most commonly as duroquinone, reflecting its origin from durene. Other listings use DQ, TMQ, or its systematic name 2,3,5,6-tetramethyl-1,4-benzoquinone. For anyone searching databases or reading older papers, keeping these synonyms in mind avoids missing key results or supplier references. The robust array of trade names and abbreviations in global distribution also reflects the compound’s diverse adoption by different language and market contexts.
Safe handling of tetramethyl-1,4-benzoquinone comes down to basic respect for its redox activity and irritant potential. Skin and eye protection prove essential, along with work in a well-ventilated hood. Direct inhalation of its vapors or dust risks irritation, and repeated exposure can sensitize workers. Waste disposal involves specialized protocols due to its persistent nature and toxicity to aquatic systems. Over years of lab routines, I’ve come to appreciate the wisdom in double-bagging waste and closely monitoring stock inventory, keeping quantities just big enough for scheduled projects to minimize hazards and regulatory headaches.
This molecule has carved a home in diverse sectors that extend from organic synthesis to specialty materials. Within the laboratory, it has been indispensable as an oxidizing agent in selective synthesis, particularly in the formation of quinones, hydroquinones, or other redox-active intermediates. In electronics, its redox cycling enables its use as a charge-transport material or as a test molecule to probe electron transfer in emerging device designs. Environmental scientists study its behavior in advanced oxidation processes as part of pollutant degradation systems. My personal involvement lies in catalytic methodology development, where the stable yet responsive quinone scaffold serves as an instructive platform for designing new catalysts or mimicking biological redox processes.
Active research drills deep into the interaction of tetramethyl-1,4-benzoquinone with both transition metals and organocatalysts, chasing new bond-forming strategies for sustainable chemistry. Studies focus on refining its synthesis for lower environmental impact, higher atom efficiency, and safer product isolation. The push for greener routes leans on biomimetic processes that echo natural metabolic cycles, offering hope for less toxic waste streams and renewable feedstocks. Its role in redox flow batteries continues to attract new collaborators from both academic and tech sectors. Having seen promising student projects spun out from modest ideas using this molecule, I know firsthand the importance of accessible compounds in fueling both foundational and applied research.
Like many small organic molecules, tetramethyl-1,4-benzoquinone brings health concerns if mishandled. Acute exposures may irritate skin, eyes, and respiratory systems, and chronic or large-scale spills threaten aquatic habitats. Studies in animal models highlight dose-dependent effects, with high concentrations potentially impacting liver function. Environmental fate and bioaccumulation rates prompt regular review by regulatory agencies, urging better containment and waste minimization. Personal training sessions, emphasizing gloves, eye shields, and strict adherence to storage guidelines, build a culture of collective safety conscience across labs. Engaging in transparent reporting and publishing exposure data strengthens community understanding and helps create more balanced safety standards for wide adoption.
The future lies in expanding tetramethyl-1,4-benzoquinone’s toolkit role into next-generation technologies. New research eyes its use in sustainable catalysis, sensor applications, and organic energy storage materials. As industries chase high-performance composites and greener electronics, the compound’s stability and manageable redox window offer ready advantages. Small tweaks to the structure could unlock entirely new applications, especially in fields like artificial photosynthesis and environmental remediation. By sharing protocols, safety insights, and application notes, the chemical community continues to push boundaries, ensuring this classic molecule remains a valuable building block for both current solutions and upcoming breakthroughs.
Tetramethyl-1,4-benzoquinone might not make headlines, but its work behind the scenes shapes more fields than most people realize. This compound belongs to a family known for electron-shuttling powers. Industries like rubber, plastics, and pharmaceuticals constantly lean on these reliable oxidants to move their processes forward. Growing up in a family of science teachers, I remember seeing old bottles of it in chemical storage cabinets—testament to how long it's played a part in labs around the world.
In the rubber industry, this compound stands out during the polymerization of synthetic rubbers. Chemists often use tetramethyl-1,4-benzoquinone as a catalyst during the manufacture of specialty rubber grades. With my own hands, I’ve watched it push a reaction along in organic labs, wrangling electrons where none of the gentler tricks worked. Its oxidative punch makes it a unique problem solver when dealing with molecules that just won't budge.
Pharmaceutical labs don’t ignore this compound either. It helps drive forward vital oxidations, allowing scientists to build active pharmaceutical ingredients with better yields and fewer steps. For example, in the synthesis of antibiotics and anti-cancer agents, the steps sped up by tetramethyl-1,4-benzoquinone often mean lower costs and improved access to medicines. The pharmaceutical world values reliable, predictable chemicals. This compound delivers both.
Every chemical comes with trade-offs. The strong oxidizing power here can mean trouble in the wrong hands or sloppy setups. From what I’ve seen, safety data sheets for tetramethyl-1,4-benzoquinone don’t get filed away in a drawer—they’re always close by. Lab workers check skin contact risks, inhalation warnings, and proper waste disposal protocols. Environmental experts point out the need for better recovery and waste treatment strategies involving these quinones. Some companies now explore greener synthesis techniques or closed-loop systems to curb the compound’s environmental impact.
Global demand for sustainable chemical processes keeps increasing. Chemists working in academia and industry both push to use less hazardous substitutes or seek ways to recycle tetramethyl-1,4-benzoquinone more effectively. In my graduate work, one team experimented with enzyme-promoted oxidations to cut down on synthetic quinones altogether, taking a cue from nature. Others in the field try to pull back waste and reclaim more chemicals for reuse, reflecting public pressure for safer, more sustainable factories.
Tetramethyl-1,4-benzoquinone highlights how one molecule can touch so many aspects of modern life—from the rubber in tires to the drugs in hospitals. Making sure that its manufacture and use keep pace with growing safety and sustainability expectations sits high on many project planners’ checklists. With the right mix of research, updated protocols, and open communication, it’s possible to hang on to chemical workhorses like this compound while cutting the risks down to size.
Tetramethyl-1,4-Benzoquinone, used in organic synthesis and dye production, demands respect. Breathing in its dust can quickly irritate the throat or nose. Skin contact stings, even resulting in painful rashes after a few fleeting moments. I once worked in a research lab where a colleague wiped a spill with bare hands. Before lunch, his skin had turned red and sensitive. That mistake stuck with our team for months.
Let’s look at gear. Splash goggles stay on through cleanup and mixing. Everyone has stories about “just a quick transfer”—one moment of lazy practice, and small droplets sneak onto eyelashes or under sleeves. The recommended gloves are nitrile or butyl rubber—regular latex gloves degrade too fast against the compound. Double-glove if handling powder or spills. Always wear a long lab coat and closed shoes; chemical-resistant aprons add another layer of smart protection, especially during larger transfers.
Drop Tetramethyl-1,4-Benzoquinone in a closed space and sharp odors quickly signal trouble. Engineering controls are crucial. Work inside a certified fume hood, keeping sash between eyes and the flask. I recall a machinery room with patchy HVAC, where opening containers instantly brought headaches. In well-ventilated spaces, fumes clear out quickly, and everyone benefits. Air quality sensors and simple airflow checks—like a tissue held at the sash line—never get old.
Storing this chemical means using simple, clear labeling and secondary containment. Containers should be airtight and stored away from acids, alkalies, and anything combustible. My own lab used color-coded bins, making grab-and-go storage less tempting, so, powder doesn’t get left on open shelving. Lock away large bottles. Nobody wants to reach above shoulder level or stretch past other bottles where powder can spill.
Annual training isn’t a bureaucratic box to tick. In my experience, running practice spill responses, even as short fire drills, builds habits. Know where the eyewash and safety shower stations sit—count the steps, and check that the path isn’t blocked. I once watched a new technician waste precious time searching for eye rinse after accidental splashing. A minute’s delay can turn a treatable exposure into something life-changing.
Pouring leftover Tetramethyl-1,4-Benzoquinone down the drain is reckless. Most university waste streams offer hazardous waste bins for organic solvents and contaminated gloves. Double bag waste and keep sharps away from glassware bins. Track quantities—a forgotten beaker collecting dust at the back of the fridge often gets ignored until found by new staff. Inventory checks prevent old chemicals from sitting around, quietly decomposing or leaking.
Staying safe with Tetramethyl-1,4-Benzoquinone is less about strict checklists than everyday vigilance. Talking through near-misses during lab meetings, sharing clear tips like “keep one hand clean” or “never open outside the hood,” and celebrating zero-incident weeks all reinforce a culture where everyone owns a slice of that safety net. It’s not just about personal safety. A robust system means nobody else pays for one person’s shortcut.
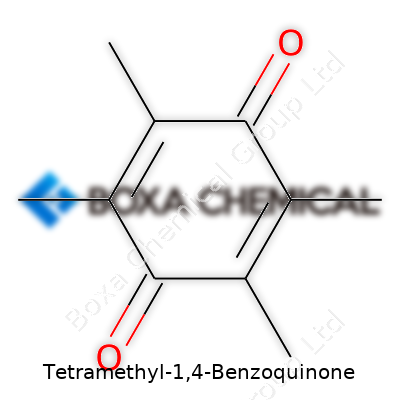
Anyone who has spent time in a chemistry lab gets used to the look of benzoquinones. Tetramethyl-1,4-benzoquinone stands out with its symmetrical design. Essentially, you take a 1,4-benzoquinone ring—think of it as a six-carbon ring broken up by two double-bonded oxygens directly across from one another—and stick four methyl groups onto it. Each of those methyls clings to a carbon next to the oxygens. The chemical formula comes out as C10H12O2, which makes sense once you visualize those extra carbons and hydrogens packed onto the quinone core. When I worked with ring-structured chemicals in grad school, adding methyls usually kicked up the volatility and changed how the molecule behaved in reactions.
If you picture a benzene ring, you’re halfway there. For Tetramethyl-1,4-benzoquinone, the double-bonded oxygens pull electrons in, making the ring more reactive. Placing a methyl at each position next to those oxygens means less space for other substitutions, and that keeps the molecule stable in certain uses. The molecular arrangement looks like this: the 1- and 4-positions hold the oxygens, and methyls take up the 2, 3, 5, and 6 spots. It’s a sort of molecular shield. This can influence its properties, like lower melting points compared to less substituted quinones or differences in color and odor.
These extra methyl groups do more than just bulk up the molecule. They help determine what kinds of reactions Tetramethyl-1,4-benzoquinone will take part in. In organic labs, methylated quinones often show up as oxidizing agents or as starting points for dyes, sensors, and more. The electron-rich methyl groups balance out the electron-withdrawing oxygens, setting up an interesting push-pull across the molecule. From firsthand experience, I know this can help chemists nudge reactions along that wouldn’t start with a bare-bones benzoquinone. It’s found use in research settings for generating radicals and as part of photochemical processes where precise reactivity matters.
Methylated quinones don’t belong in inexperienced hands. They can stain, irritate, and in the case of 1,4-benzoquinone derivatives, pose toxicity risks through inhalation or skin contact. I’ve seen the damage a small spill can do—those orange-yellow stains stick to gloves and bench tops for weeks. Lab safety requires proper ventilated hoods and nitrile gloves during use. The methyl groups may bump up vapor pressure, so airborne exposure can be a concern if you’re not careful.
In fields like organic electronics and materials science, Tetramethyl-1,4-benzoquinone’s specific chemistry supports electron-transfer reactions in ways that plainer kin can’t match. More than once, researchers have pointed to the methyl's influence on stability and solubility as crucial for results in polymer synthesis or even as prototypes for organic batteries. Proper identification—the formula C10H12O2 and the distinct ring structure—means manufacturers and labs reduce mistakes and stay compliant with environmental rules. Knowing the structure isn’t just for textbooks; in practice, it keeps workers safe and processes efficient.
Working with chemicals like Tetramethyl-1,4-Benzoquinone isn’t new for anyone with a background in a chemistry lab or research setting. This compound sees use in organic synthesis, dye manufacture, and sometimes shows up in academic projects. But as much as the name might sound like something out of a textbook, it’s the practical steps for safe storage that make the real difference.
Tetramethyl-1,4-Benzoquinone can decompose when exposed to air and moisture. I’ve seen what can happen if someone leaves a jar open just a little too long—yellow-brown crystals start losing color and form a sticky mess. If you’re not careful, an entire container might go to waste, and no one likes finding ruined quinone contaminating a shared storage shelf.
Oxygen and water aren’t just enemies in theory; oxidative degradation leads directly to chemical breakdown, and the presence of water can start unwanted reactions. Researchers and anyone using this material can avoid accidents and lost product by remembering that air-tight means just that—no lazy screw-tops and no half-closed zip bags.
People often underestimate the impact of temperature. Store this quinone at room temperature in a dry, well-ventilated space—but absolutely away from heat sources or direct sunlight. On a warm day, stuffy cabinets can become mini-ovens. That’s how someone ends up with unstable crystals and, more rarely, funky fumes. Refrigeration helps, but only when containers block out moisture too. Nobody wants a chemical bottle dripping with condensation.
Low fire risk doesn’t mean zero risk. Tetramethyl-1,4-Benzoquinone isn’t classed as explosive, but it still poses a flammability hazard. Keep it tucked away from solvents, acids, and especially strong bases—that’s just asking for trouble. Label containers clearly and check seals regularly, because one forgotten or loose lid can turn into a headache for everyone in the lab.
Direct skin contact with this quinone causes irritation fast. Anyone handling it should use gloves (nitrile beats latex here), safety glasses, and if you’re measuring out powders, do it in a fume hood. Lab coats stop dust from sticking to your own clothes. During my own years in a teaching lab, a fellow student managed to spill a bit across the bench—one slip, and cleanup took an hour. Having a spill kit nearby and using absorbent pads saves time and keeps toxins off your hands.
Routine checks catch problems before they scale up. Supervisors should walk through storage areas each week to spot leaks or damaged packaging. Digital logs for chemical inventories can flag expiry dates or unusual movement, making tracking easier. Drying agents like silica gel packs go a long way for sealed storerooms. If a batch shows signs of spoilage, flag and dispose of it safely, following hazardous waste protocols instead of dumping it in the trash.
Storing Tetramethyl-1,4-Benzoquinone safely isn’t just about avoiding mishaps—it’s about respecting both the material and the environment everyone shares. Experience in any real-world lab reinforces: safe storage starts with the basics and demands attention every single day.
Factories and research labs depend on hundreds of specialty chemicals. Tetramethyl-1,4-benzoquinone, sometimes known as duroquinone, stays mostly out of the spotlight, but it deserves more attention. It shows up in organic synthesis, polymer work, and some battery technologies. Researchers and workers who handle this compound every day carry the weight of potential health hazards, often without clear information on the dangers they might face.
Daily life rarely brings us into contact with this substance. Inside certain plants and labs, accidental spills, dust generation, or vapors can lead to inhalation and skin exposure. Wearing gloves and protective clothing at work does reduce risks, but accidents happen, and often there's a false sense of security around less-talked-about chemicals.
Scientific literature, sometimes hard to wade through, shows tetramethyl-1,4-benzoquinone’s potential to irritate eyes, skin, and respiratory passageways. Breathing in dust or vapors may bring on coughing, wheezing, and shortness of breath. Contact with skin or eyes could cause burns or itching. I once saw a coworker develop a nasty rash from failing to spot a ripped glove during clean-up; that memory sticks when considering the need for better handling procedures.
The story gets more serious with longer exposure. Benzoquinone compounds belong to a family of chemicals that can damage cells through oxidative stress. Researchers warn that some quinones may affect DNA or lead to changes in blood cells. High levels bring more danger, but even routine handling without precautions can lead to chronic issues. Animal studies raise alarm bells, though finding human-focused, large-scale data remains a challenge.
Workers often shrug off minor headaches or mild breathing trouble, chalking them up to dust, fatigue, or bad ventilation. Over time, this small stuff adds up. The nervous system can face subtle hits from repeated chemical exposure, sometimes leading to symptoms that show up long after the workday ends. Years ago, I learned that regular health surveillance and honest talk in the break room can bring invisible hazards to light—stories about nosebleeds, sore throats, and lingering fatigue often preceded any official documentation of chemical hazards in workplace audits.
Good ventilation, regular safety audits, and simple hygiene stand as the best shields. I’ve found that managers who empower workers with real knowledge and open reporting tend to face fewer health issues over time. Fixing leaks, installing better exhaust systems, and enforcing glove changes make a visible difference. Training doesn’t work unless workers actually believe the risks matter, so sharing real-world examples resonates more than warnings buried in safety data sheets.
Chemical safety isn’t just about following rules. It involves passing down stories about narrow misses, seeking out new research, and changing habits that feel safe only through routine. Tetramethyl-1,4-benzoquinone might never become a household name, but every industry using it should break the silence about its hazards. Open communication and continuous education offer the real path to protecting everyone’s health at work.
| Names | |
| Preferred IUPAC name | 2,3,5,6-tetramethylcyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˌtɛtrəˈmɛθɪl wʌn fɔː ˌbɛnzəʊkwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 527-17-3 |
| Beilstein Reference | 1720294 |
| ChEBI | CHEBI:45689 |
| ChEMBL | CHEMBL109695 |
| ChemSpider | 12318 |
| DrugBank | DB04220 |
| ECHA InfoCard | 01-2119565153-38-0000 |
| EC Number | 203-710-6 |
| Gmelin Reference | 8488 |
| KEGG | C06504 |
| MeSH | D014964 |
| PubChem CID | 6977 |
| RTECS number | DQ9625000 |
| UNII | GYA4Y8S09W |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8033547 |
| Properties | |
| Chemical formula | C10H12O2 |
| Molar mass | 164.20 g/mol |
| Appearance | Yellow crystals |
| Odor | Pungent |
| Density | 1.09 g/cm³ |
| Solubility in water | Moderately soluble |
| log P | 1.6 |
| Vapor pressure | 0.9 mmHg (20 °C) |
| Acidity (pKa) | -0.7 |
| Basicity (pKb) | 6.56 |
| Magnetic susceptibility (χ) | -37.2e-6 cm³/mol |
| Refractive index (nD) | n20/D 1.544 |
| Viscosity | 15.8 mPa·s (25 °C) |
| Dipole moment | 2.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 302.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -150.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1362.4 kJ/mol |
| Pharmacology | |
| ATC code | N07XX |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye damage, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P210, P261, P264, P271, P301+P312, P304+P340, P305+P351+P338, P312, P330, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-2 |
| Flash point | 52 °C (closed cup) |
| Autoignition temperature | 716 °F (380 °C) |
| Lethal dose or concentration | LD50 oral rat 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1300 mg/kg (oral, rat) |
| NIOSH | DH6650000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m3 |
| IDLH (Immediate danger) | 50 mg/m3 |