Sodium cresolate emerged during the late 19th and early 20th centuries, growing out of a period when chemistry rapidly transformed industry and medicine. The pursuit of more potent disinfectants drove researchers to experiment with coal tar derivatives. Phenol set the pace, but its close relatives, the cresols, soon followed. Mildly improved safety, better handling, and broader antimicrobial action convinced both scientists and industrialists. Soon, sodium cresolate found a home in public health, livestock farming, and even wartime sanitation. Long before modern antibiotics, rural clinics depended on cresolate-based solutions to keep wounds and surgical tools clean. Old government reports and technical journals show a clear pattern: innovation by necessity, adaptation by experience. With each decade, improved production methods and sharper regulations changed both the way sodium cresolate was made and where it was used.
Sodium cresolate comes across as a dark brown liquid or solid, produced through the neutralization of cresols with sodium hydroxide. It carries a sharp, almost medicinal odor, familiar to anyone who’s scrubbed a barn wall or worked with classic cleaning fluids. Manufacturers offer a range of concentrations, but most commercial products fall between 50% and 80% sodium cresolate in water or a solvent mix. Its efficiency as an antiseptic and deodorizer isn’t just folklore. Hospitals, schools, animal quarters, and transportation hubs used sodium cresolate for over a century, thanks to its broad spectrum of action and practical availability. Chemists depend on it for standardization. Few alternatives compete on price or time-tested reliability, though stricter rules and emerging green alternatives are catching up.
The properties of sodium cresolate give real insight into why it’s stuck around. Water solubility stands high, making it easy to use for surface cleaning or machine wash-downs. Under ordinary conditions, the compound clings to a deep brown shade, sometimes hinting at green or red, depending on impurities and the batch. The liquid form proves caustic to the touch, so thick gloves go on before work. Chemically, it acts as a moderately strong base and reacts with acids to release free cresols. Sodium cresolate holds up well under normal storage, rarely breaking down, though some formulations can separate or evaporate over time. It handles temperature swings decently, too, with its key properties staying stable above freezing and up to moderate heat, so long as containers stay closed. The compound shows low volatility, so accidental inhalation happens mostly during spills or careless mixing.
Standards for sodium cresolate draw from decades of safety reviews and practical experience. Labels on containers call out the main hazards—corrosiveness, toxic vapors, need for protection. Producers follow both local and international guidance, such as GHS or OSHA, to include warnings about ingestion, skin contact, and what to do in emergencies. Specifications tell buyers about cresol content, the sodium fraction, water percentage, and limits on undesirable side-products: too much phenol or leftover lye, for example, can cut performance or increase risks. Many suppliers run detailed batch tests and publish results online. Labels tell a lot about how an industry values honesty and safety—claims get tested, and buyers demand proof. Some companies include traceability codes, so anybody handling a leak or exposure can check the details and get advice.
Producing sodium cresolate starts with crude cresol, extracted from coal tar during distillation. Chemical engineers mix cresol with sodium hydroxide under tight temperature control. The reaction yields sodium cresolate and water, which settles out if the product holds enough solids. Industrial processes run this in large kettles, often under ventilation to keep fumes under control and staff safe. Purification involves filtering out tarry residues, skimming off excess liquid, and sometimes passing the batch through activated carbon. With automation, yields climbed and energy costs dropped, but skilled operators still watch for tricky reactions and inconsistent feedstock. Old-line chemical plants still stick to time-proven equipment, finding that modest upgrades work better than disruptive overhauls. Most firms bottle product directly from the reactor or holding tank, minimizing exposure and inconsistency. Waste streams call for careful neutralization or incineration, limiting the environmental footprint.
Sodium cresolate’s reactivity offers both benefits and challenges to chemists. It reacts quickly with acidic substances, breaking down into cresol and the corresponding neutral salt. Mixed with chlorine or strong oxidizers, the compound generates a flurry of byproducts—some useful, some hazardous—requiring good ventilation and plenty of attention to detail. Research labs explore modifications to improve selectivity, increase germ-killing power, or reduce odors and toxicity. Sulfation, ethoxylation, and the addition of surfactants give custom blends that tackle special cleaning jobs or lower the overall risk profile. Not every tweak works out; testing can show side effects like skin irritation or lingering residues. Yet broad experience in the field means that most changes get tested in small pilot batches before wider use. Transparent reporting of chemical changes helps emergency responders deal effectively with accidents or misuse.
Anyone searching industry catalogs for sodium cresolate stumbles onto a raft of alternate names: sodium cresylate, sodium o-cresolate, sodium p-cresolate, or simply sodium cresols. Trade names from the early days—Lysol, Creolin, or Jeyes Fluid—carry echoes of a time when public health ran hand-in-hand with innovation. Technical manuals sometimes use formal nomenclature: sodium (methylphenol)ate, cresylic acid sodium salt, or sodium alkyl phenolate. Legacy naming can trip up new entrants; changes over the years reflect both evolving standards and minds shifting toward clearer hazard communication. Different regions stick to their favorites, but almost every supplier provides cross-references so users can match up local and international standards without confusion.
Safe handling of sodium cresolate draws from real-world mistakes and lessons learned, not just textbooks. Chronic skin irritation and dangerous fumes claimed more than a few early workers who brushed off the warnings. Now, up-to-date facilities rely on training, good ventilation, proper gloves, and eye protection. Accidental spills get neutralized with dilute acid and plenty of water, then mopped up with absorbent materials for secure disposal. Companies invest in spill kits and emergency showers, not only for compliance, but out of hard-won caution. Countries draw guidance from organizations like the CDC, EPA, and ECHA, which all catalogue incidents and promote improvements. Monitoring exposure with badges or periodic medical checks caught on after some high-profile accidents in the twentieth century. Storage rooms carry prominent signs about no eating, drinking, or smoking near sodium cresolate, part of an overall defensive posture that reflects deep respect for both the chemical and its hazards.
Sodium cresolate proves its value in some of the toughest cleaning and disinfection challenges. Farmers rely on it for sanitizing animal enclosures, where microbes and parasites threaten livestock. Public transit systems turn to it during outbreaks, where hard surfaces and high touch points need something that actually works. Food processing plants once depended on cresolate-rich cleaners to tackle greasy buildup and persistent bacteria, though stricter food safety regulations send them searching for lower-residue options now. In oil and gas, sodium cresolate has carved out a niche breaking down stubborn organic deposits and in certain drilling fluids. Museums and libraries rarely use it due to risks to health and artifacts, but it does find occasional use in pest control or mold cleanup. Industrial laundries mix cresolate into some formulations targeting tough stains and odors, a practice with roots stretching back decades. Though many newer options arise, sodium cresolate’s combination of cost, effectiveness, and simplicity still speaks to managers facing tight budgets and real cleaning needs.
The world of sodium cresolate research runs deep, from fundamental chemistry to day-to-day problem-solving. Academics probe reaction mechanisms, proposing tweaks that might lower toxicity or boost antimicrobial power. Industry scientists experiment with blend ratios, stabilizers, and additives to stretch product shelf life and cut down on hazardous breakdown products. Independent labs look for greener routes: bio-based cresols or catalytic methods that shave energy off the process. The steady pressure from environmental rules and worker safety shines a light on even minor improvements. New studies compare sodium cresolate head-to-head with quaternary ammonium compounds, hydrogen peroxide, or chlorine— sometimes showing old chemicals holding their ground, other times revealing weak spots. Feedback loops tie together field results, regulatory findings, and chemical tweaks, fostering a gradual climb toward safer, more sustainable use. The best labs encourage direct communication with emergency personnel, environmental watchdogs, and end users to ground research in lived experience.
Toxicologists keep a close eye on sodium cresolate. The compound’s effectiveness comes with real risks: exposure can burn skin, irritate eyes, or in the worst cases, cause liver, kidney, or central nervous system damage with enough uptake. Repeated contact sometimes triggers allergic skin reactions or chronic dermatitis, especially in people lacking protective gear. Inhalation of high concentrations causes coughing, shortness of breath, and occasionally, lasting respiratory harm. Laboratory animal studies echo these findings and point to moderate acute toxicity, though less severe than pure phenol. Environmental scientists flag sodium cresolate for aquatic toxicity, especially in concentrated waste streams. Real-world poisonings remain rare, mostly due to strong safety culture and clear labeling. Manufacturers reduce risk by limiting concentrations, improving packaging, and offering extensive training. Chronic exposure studies remain ongoing, as regulators work to define safe occupational thresholds and updated cleanup guidelines.
Sodium cresolate stands at a crossroads between tradition and innovation. New regulations and public perceptions pull industries toward safer, biodegradable alternatives—especially in food, health care, and environments with sensitive populations. Engineers weigh energy savings and process tweaks, searching for ways to cut emissions or reclaim spent chemicals before they hit the wastewater stream. Pressure from NGOs and consumer advocacy groups pushes large corporations to revisit their entire cleaning product lineup, swapping out old ingredients for modern blends. Despite these trends, many organizations—especially in developing markets—stick with what they know for reasons of cost, availability, or local regulations. The challenge lies in managing the transition: clear communication, robust retraining, and reliable data arm decision-makers with what they need to carry changes through without introducing new risks. Smart research could open paths toward less toxic, plant-based antimicrobials or even clever recycling loops to recover cresols from waste. The story of sodium cresolate’s next century looks set to blend history, necessity, and forward-thinking science.
Sodium cresolate isn’t a household phrase, but its job keeps quite a few industries humming along. Walk into a hospital or a food processing plant, and the odds are high you’ll find products cleaned using some blend that counts sodium cresolate as an ingredient. Its punch as a disinfectant gets put to work scrubbing away bacteria, fungi, and viruses. That makes it an old favorite for places where keeping germs at bay takes priority.
Growing up in a small farming town, I saw livestock pens cleaned with strong-smelling liquids. Turns out, many of these solutions contain sodium cresolate, which helps protect animals from disease outbreaks. Around animal shelters and barns, staff trust it to clear out disease-causing organisms. Every farmer I know wants tools that work—and not just halfway. As a phenolic compound, sodium cresolate gets deep into surfaces and helps keep spaces a little safer, especially during seasons when livestock illnesses threaten entire herds or flocks.
Factories and garages see grease, oil, and tough stains build up fast. Sodium cresolate joins the roster in heavy-duty degreasers. Whether it’s a car mechanic faced with engine grime or a worker in a metal shop, this cleaner breaks down sticky messes. That same grease-busting action gets harnessed in shipyards, maintenance bays, and any setting where metals meet machinery.
My uncle worked on trains for decades. By the end of his shift, the tools and shop floor always looked like a battle zone of grease and oil. Their strongest cleaner, he said, smelled “sharp” and cleaned “like nothing else”—turns out, sodium cresolate helped cut through that buildup every day.
Sodium cresolate packs plenty of cleaning firepower, but it comes with tradeoffs. If you’ve ever opened a container and caught the chemical stink, you know to step back or put on gloves. Skin contact can cause burns, and breathing the fumes can irritate the lungs. In food processing, if rinsing leaves any residue, there’s a risk for contamination. The EPA lists cresols as hazardous waste that needs careful handling. In my early days working at a feed mill, hazmat training always stressed not just how to clean, but how to protect yourself, too.
Society keeps finding new ways to protect people and the environment while still fighting bacteria and grime. Safer alternatives keep showing up on the market. Some facilities choose hydrogen peroxide-based solutions or enzymatic cleaners, especially if staff health takes top priority. Regulations have encouraged businesses to rethink cleaning protocols and look for low-toxicity swaps, especially anywhere food or medicine gets processed. Still, the raw effectiveness of sodium cresolate remains tough to beat in some scenarios.
Knowing where sodium cresolate fits—and where it doesn’t—helps communities and businesses strike a balance. The cleaning industry keeps pushing for agents that protect health without harming workers or surrounding ecosystems. Every new rule or alternative brings a wider view, but that original phenolic backbone paved by sodium cresolate still matters in certain situations. As information gets shared between industries and from scientists to the folks actually using these chemicals, smart choices get easier. That’s the kind of progress that goes beyond shiny labels and dives into real-world impact.
Sodium cresolate lands in a strange space between useful and risky. This chemical blend shows up mainly as a cleaning agent, famed for its ability to cut through grime, disinfect barn floors, and keep industrial workspaces free from harmful bacteria. Workers in agriculture, factories, and healthcare sometimes cross paths with it, often without realizing the risk that comes with casual handling.
Sodium cresolate does more than banish dirt; it carries a punch that demands respect. Anyone splashing the concentrate on bare skin feels burns or irritation almost immediately. Breathing in vapors sparks coughing, headache, nausea, or — if unlucky — even more serious lung effects. Out of curiosity, I once checked out the safety sheet in a warehouse. Right at the top: “Harmful if inhaled. Avoid contact with skin and eyes.” Not exactly comforting words. That warning didn’t pop out of thin air. Real emergencies happen. Burns on workers' hands and accidental eye splashes have sent people straight to the ER. The U.S. National Institute for Occupational Safety and Health (NIOSH) lists sodium cresolate as a hazardous substance with clear exposure limits. Swallowing can shut down organs. Even diluted forms can trouble skin, especially over time.
The tough part: people often grow numb to labels. When jobs demand quick turnaround, gear gets skipped, gloves forgotten, and ventilation suffers for the sake of speed. And yet, consequences don’t go away. I learned this truth early while working with janitorial crews in old-school school buildings. Cutting corners on gear led to rashes and heavy coughs. Later, stories of chronic lung issues or persistent skin problems weren’t rare. Big industries face the same constraints, just on a larger scale.
Knowledge changes everything. Simple habits help: reading the label, double-checking the chemical's Safety Data Sheet, and grabbing the right gloves — not any old pair but chemical-resistant ones. Splash goggles beat plain safety glasses. People often undervalue real ventilation, yet opening more windows or switching on exhaust fans keeps vapors on the run. Keeping washing stations close by isn’t overkill. One splash and access to running water can stop a hospital trip. In my own experience, the places that trained their teams, posted reminders, and didn’t cheap out on the basics saw fewer problems.
Industry groups push for more transparency, and governments continue updating hazard communication standards. For example, OSHA’s Hazard Communication Standard now expects employers to train workers before using such chemicals, not after a problem flares up. This proactive approach helps, though enforcement can lag in busy workplaces. At schools, hospitals, and warehouses, managers who walk their talk make the biggest difference. We've all seen the aftermath of poor chemical handling and know grudges against “too much regulation” never hold up when someone gets hurt.
Looking at how things work in the real world, sodium cresolate belongs in a locked cabinet, not in the open. Regular check-ins and a little extra investment in safety supplies pay for themselves. The rule is simple: bad things happen when safety turns into an afterthought. Encouraging shared knowledge, peer reminders, and basic caution beats any clever slogan. Workers hold the power to keep themselves and their team healthy. Respecting what sodium cresolate can do, both good and bad, lands us on the right side of safety.
Sodium cresolate plays a key role in industrial cleaning, disinfectants, and some specialty chemical processes. The compound takes the form of a yellowish to brownish liquid or powder, and anyone who works with it knows it puts off a sharp, phenolic odor. Not something you want floating through your workspace. Its caustic and corrosive traits mean careless storage invites not just mess, but harm.
Straight talk: store sodium cresolate in a place that keeps people and the environment safe. I’ve seen workplaces where improper chemical storage invites accidents. To cut that risk, keep the temperature stable. Too much moisture causes clumping or even leakage; too much heat ups the odds of pressure build-up in storage drums. In my old lab, we installed clear ventilation and temperature controls just to head off these issues.
Sodium cresolate attacks certain metals. Use containers built from compatible materials—high-density polyethylene, strong stainless steel, or glass-lined vessels. Skip out on aluminum and regular steel since the compound eats right through them in no time. Rust, leaks, and broken seals lead to major headaches and unsafe working conditions. I’ve seen a busted drum turn a safe, organized storeroom into something needing a hazmat call.
A chemical like this demands sturdy shelving and secondary containment trays. In one plant, we had strict rules: all caustic agents like sodium cresolate sat on lower shelves with trays underneath, so a spill stopped before spreading. Not only does this reduce slip risks, but environmental protection agencies expect it.
Label containers with bold, clear words stating the chemical name and hazard class. Someone once mislabeled a drum, and the next shift got a nasty scare when opening it. No shortcuts—put safety pictograms, emergency contacts, and handling instructions right on the container.
Sodium cresolate isn’t just another cleaning agent. Breathing its fumes or getting it on your skin can cause burns and respiratory problems. This isn’t fearmongering—OSHA data backs it up. Our team always stored it away from acids and oxidizers. Mixing these by mistake kicks off violent reactions and deadly fumes. Keep neutralizing agents handy—something like sodium bicarbonate allows quick action after accidental spills.
I always push for regular safety drills, making sure staff can use spill kits and protective gear. Even storage areas need good ventilation, not just work zones. Proper ventilation draws away vapors, reducing the buildup of harmful concentrations.
Improper storage creates chemical runoff, damaging soil and water supplies. Regulations exist for a reason. Local authorities perform unannounced spot checks, and fines for noncompliance hit hard. My last employer nearly lost key contracts after a minor leak made the evening news. Investing in secure storage kept the facility open and protected the community.
Old stock shouldn’t gather dust. Regular checks and inventory management cycle aging product out safely, cutting down on hazardous waste. This kind of discipline saves money, reduces risk, and builds trust.
Storing sodium cresolate right goes beyond rule following; it’s a duty to colleagues, neighbors, and the company’s bottom line. It takes knowledge, attention, and the willingness to go the extra mile each day.
Before tossing any sodium cresolate in a bin, it’s worth remembering how easy it is for strong chemicals to slip into water, soil, and air, doing real damage if ignored. Sodium cresolate goes beyond being just another industrial cleaning chemical. Its strong, toxic properties can harm both people and animals. The same chemical that wipes out stubborn grime at a plant can burn skin, irritate lungs, and poison waterways if it slips through careless hands.
I’ve seen a few workshops cut corners to save on waste hauling. A few flushes down an old drain, and everything seems out of sight, out of mind. That thinking comes back to haunt you. Fish kills in local streams, sick workers with respiratory trouble, and local fines will show up. The right way costs a little now, but disasters always cost more a few years down the line. Data from EPA reports show improper disposal is behind spikes in pollution complaints in communities near factories.
Plenty of folks believe sodium cresolate safely breaks down if you just dilute it. That’s wishful thinking. Sodium cresolate sticks around—leaching into groundwater or spreading airborne as fumes, especially if handled by people who don’t know the rules. EPA classifies it as hazardous waste for a reason. Mixing it with general trash or pouring it down the drain isn’t just illegal; it’s dangerous for everyone.
Personal experience with chemical storage shows that even minor leaks or spills give a punchy, sharp smell that lingers. That smell signals health risks, not just inconvenience. Given U.S. Department of Transportation guidelines, facilities working with sodium cresolate have clear instructions. Turn to an experienced hazardous waste coordinator, and you’ll see heavy labeling, locked cabinets, spill response kits, and strict procedures for dealing with even small residues.
The ideal approach draws from both regulation and common sense. Separate sodium cresolate from other waste streams in chemically-resistant containers. Label everything. Keep those containers closed tight except during use. Every facility using sodium cresolate should work with a certified hazardous waste handler. Good disposal outfits know how to neutralize, incinerate, or treat sodium cresolate waste, preventing it from hitting the open environment. Contracts with certified transporters and disposal companies prevent regulatory headaches and environmental incidents.
Local regulations matter just as much as federal law. Most towns require chemical waste tracking paperwork from the point it leaves a building until final treatment. Document every step. Rely on storage containers meeting EPA and Department of Transportation specs.
Smaller operations sometimes struggle to keep up due to cost. Regional governments and industry groups can offer step-by-step guides. Many states provide hazardous waste amnesty days, where professionals collect small business and residential hazardous chemicals safely. These programs open the door for proper disposal without heavy burdens on small firms or households.
Routine training for staff handling sodium cresolate stops mistakes early. Employees who understand both the dangers and the proper steps prevent accidents before they start. On a practical level: don’t improvise. Call a professional. Take every warning on the label seriously, and remember that the cost of safety is always less than the price of ignoring the risk.
Community protection starts with every drum, every label, and every decision to take disposal seriously.Walking into an industrial site or a cleaning supply warehouse, most people probably don’t give a second thought to sodium cresolate. This chemical finds use in disinfectants, wood preservatives, and even certain degreasers. For folks on the front lines—janitors, factory workers, farmers—it’s a real-world risk with very real health impacts. Once in the air or spilled across surfaces, sodium cresolate stays tough to contain, making direct and indirect exposure a stubborn safety problem.
People breathe fumes or touch contaminated equipment. Inhaling sodium cresolate can hit the lungs hard, leading to coughing, chest tightness, and wheezing. In my time working in a storage facility with powerful cleaning agents, I saw coworkers battle frequent headaches and sore throats after repeated handling. Even a small splash on the skin sets off burning, redness, and painful blisters—a sure sign the chemical breaks down skin’s natural barriers in no time.
Health risks extend past obvious irritation. Repeated or heavy exposure leads to dizziness, confusion, and in some cases, liver and kidney issues. The National Institute for Occupational Safety and Health (NIOSH) links high-level exposure to dangerous organ damage and central nervous system depression, including poor coordination and judgment. There’s never any warning before those symptoms start. On a busy shift, no one wants to wait for signs—a small dose can turn into a big problem.
Priority organs like the liver and kidneys process everything you touch or breathe in; sodium cresolate, because of its corrosive nature, forces these organs to work overtime. Lab tests in animal studies point to long-term risks, including chronic bronchitis and reduced immune function, when the body gets zero break from the chemical. That gets personal fast—for workers without prompt access to medical care, a week of exposure can lead to months of recovery.
Tightening safety practices hands workers what they need: gloves, goggles, and better ventilation. Having spent years around harsh chemicals, I learned the importance of deep-clean showers and quick glove changes. That small shift in habit often prevents the sting and redness that lingers for hours after a spill. Management has to back up those habits, giving clear information and fast access to wash stations.
Even at the regulatory level, oversight slips when checks come few and far between. Stronger workplace inspections and training programs give teams the knowledge to spot danger and react fast. Product labels need plain, direct warnings—not buried technical language. Union efforts and advocacy groups could push for more data reporting, making it easier for families and communities to know when there’s a risk.
Big industrial brands and smaller cleaning crews both face the challenge of handling sodium cresolate safely. Solutions don’t always cost much—sometimes, it’s about the right mask, the right sign, and a culture that treats safety as a shared responsibility. Though sodium cresolate comes with a laundry list of risks, better information and straightforward safety steps help keep workers out of the emergency room and on the job.
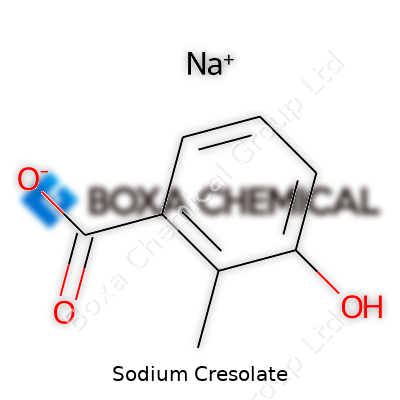
| Names | |
| Preferred IUPAC name | sodium 4-methylphenoxide |
| Other names |
Cresylic acid sodium salt Sodium cresylate Sodium cresotate Cresol sodium salt Sodium cresol |
| Pronunciation | /ˈsoʊdiəm ˈkrɛsəˌleɪt/ |
| Identifiers | |
| CAS Number | 1319-77-3 |
| Beilstein Reference | 1491327 |
| ChEBI | CHEBI:63122 |
| ChEMBL | CHEMBL64007 |
| ChemSpider | 12031 |
| DrugBank | DB11130 |
| ECHA InfoCard | 100.028.049 |
| EC Number | 215-185-5 |
| Gmelin Reference | 6074 |
| KEGG | C01762 |
| MeSH | D013052 |
| PubChem CID | 86984 |
| RTECS number | GO9260000 |
| UNII | 59XQ4HI3AO |
| UN number | UN2922 |
| Properties | |
| Chemical formula | NaOC₇H₇ |
| Molar mass | 180.18 g/mol |
| Appearance | Dark brown liquid |
| Odor | phenolic |
| Density | 1.18 g/cm³ |
| Solubility in water | soluble |
| log P | -1.7 |
| Vapor pressure | <0.1 mmHg (20°C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 10.26 |
| Magnetic susceptibility (χ) | -37.0e-6 cm³/mol |
| Refractive index (nD) | 1.54 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 99.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -470.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3919 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D08AL03 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314, H318, H410 |
| Precautionary statements | P260, P264, P270, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P362+P364, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-1 |
| Flash point | 112°C |
| Autoignition temperature | 540°C (1004°F) |
| Lethal dose or concentration | LD50 (oral, rat): 520 mg/kg |
| LD50 (median dose) | LD50 (median dose): 520 mg/kg (oral, rat) |
| NIOSH | WI5690000 |
| PEL (Permissible) | PEL: 5 mg/m3 |
| REL (Recommended) | 300 mg/m³ |
| IDLH (Immediate danger) | 250 mg/m3 |
| Related compounds | |
| Related compounds |
Cresol Potassium cresolate Sodium phenolate Sodium cumenate Sodium xylenolate |