Resorcinol Monoacetate traces its roots to the dusty shelves of early 20th-century chemistry labs. Early researchers sought alternatives to basic resorcinol, especially as new antiseptics and topical formulations grabbed the spotlight. Back then, chemists had a knack for transforming familiar substances using acetylation, a staple process in organic synthesis circles. Resorcinol, known since the late 1800s, eventually gained an acetate ester thanks to the persistent search for less irritating skin antiseptics and intermediates for more complex compounds. Medical journals from the 1920s sprinkled references to monoacetate derivatives, flagging them as less caustic than raw resorcinol. Over the decades, this compound stuck around in pharmacopoeias and research as new uses kept popping up.
You will find Resorcinol Monoacetate in the form of a pale yellow crystalline powder, slightly tacky when left out in humid air. The compound holds a place both in labs and industry, serving as a topical antiseptic, a polymer intermediate, and an occasional photographic chemical. In pharmacy counters, ointments and salves for skin disorders often list it, especially in products aimed at stubborn acne or eczema. Chemists appreciate its reactivity and the balance between solubility and stability. Bulk containers shipped to formulators sport clear hazard designs and barcode-tracked labeling, ensuring traceability right from the synthesis vat to the retail shelf.
Here’s the kind of information you see on a long-handled bottle in a chemical storeroom: Resorcinol Monoacetate crystallizes in fine needles or flaky sheets, giving it a high surface area. The melting point sits around 143°C, a little lower if mixed with residual moisture or stored poorly. Its solubility varies: dissolves well in ethanol and ether, less impressive in plain water. The acetate group tweaks its polarity, masking some reactivity of the parent phenol. On the chemical stability front, proper low-humidity storage keeps it stable for years, but light and air hasten oxidation, turning exposed samples gradually brown.
Suppliers set minimum purity at 98%, with typical impurity profiles including leftover acetic acid or traces of diacetate. Packages usually display the product name, net weight, batch code, primary chemical hazard (eye and skin irritation), and a requirement for gloves and eye protection on handling. Manufacturers keep an eye on residual solvents due to stricter EU and FDA standards, and new safety data sheets now point out the material’s dust explosion risk, a nod to safety accidents from the past two decades. Older labels often missed out on storage temperature warnings, but modern packaging includes “keep cool and dry, out of direct sunlight”.
Lab workers generally take resorcinol and treat it with acetic anhydride in the presence of a mild acid or base catalyst. The reaction stirs at room temperature or a little above, noticed by a faint vinegar smell from liberated acetic acid. Later, the reaction mix gets poured into a large volume of ice-cold water, causing the monoacetate to crystallize. This crystallized product undergoes repeated washing and vacuum drying to reach technical purity. The process seems straightforward but scale-up poses challenges—getting precise acetylation without overreaction to the diacetate calls for plenty of trial batches and tight process monitoring.
Chemists like to tinker with resorcinol monoacetate for further acetylation, oxidation, or halogenation. The acetate group brings enough stability to allow selective reactions elsewhere on the ring. Under acidic hydrolysis, the compound flips back to resorcinol and acetic acid, a useful feature in environmental cleanup and in clinical metabolite studies. Nitration and sulfonation proceed slower than with raw resorcinol, and the acetate ester gets in the way of certain coupling reactions, often forcing chemists to strip it off or protect other positions beforehand. Some research papers report attaching longer acyl chains to temper reactivity further, eyeing specialty polymer use.
Resorcinol Monoacetate shows up under a tangle of names, causing confusion in procurement unless you stick with CAS# 102-29-4. Official labels read “1,3-Benzenediol monoacetate,” “monacetylresorcinol,” or “resorcinyl acetate.” Dermatology circles grab creams and ointments calling it “Resacetine” or “Resacet.” Even international regulatory filings don’t keep standardized titles, adding to the paperwork headaches for importers and pharmacists. Despite this, trained eyes can spot it by formula (C8H8O3 or C6H4(OH)OCOCH3) and cross-reference purity data, steering clear of simple resorcinol or the fully diacetylated cousin.
Resorcinol monoacetate takes a firm spot on the list of “side-eye” chemicals in industrial hygiene manuals. Handling powder or concentrated solutions comes with basic PPE requirements: gloves, goggles, and a dust mask if dumping in larger containers. Spills on skin don’t sting as much as resorcinol, but the risk of delayed irritation and uncommon but documented allergic reactions keeps companies conservative on exposure standards. Workplaces need properly vented hoods, and clean-up includes special bins rather than tossing straight into regular trash. Fire risk shows up in safety data—dust can spark fires if left in piles, pushing storage away from open flame.
A walk through any old-school pharmacy or chemistry lab often uncovers a jar of resorcinol monoacetate cream. Dermatologists reach for it to tackle chronic skin ailments, acne, and certain fungal infections. Topical salves blend it for controlled antiseptic action, appreciated in cases where plain resorcinol triggers too much irritation. Beyond medicine, chemical manufacturers use it to feed more intricate syntheses for photographic developers, polymer building blocks, and adhesive additives. The acetate ester’s footprint in these specialty products traces back to the need for both stability during mixing and predictable breakdown under mild acidic conditions.
Labs focusing on slow-release antiseptics dig into resorcinol monoacetate’s promise, tweaking its structure for even milder action or for extended release from transdermal patches. Researchers in materials science explore its potential as a monomer for niche polyesters or advanced adhesives, citing its phenolic core as a benefit to thermal stability. Publication databases log a surge of work in greener synthesis, replacing heavy chemical catalysts with bio-enzymes or recyclable solid acids. That said, regulatory researchers call for more data on byproduct profiles and downstream ecological impact.
Animal studies and clinical reports point out that resorcinol monoacetate holds up as generally safer than its parent compound when used correctly. Swallowing large amounts still causes toxic effects—nausea, headache, and rare organ impacts. Patch tests on skin suggest infrequent but present delayed allergic dermatitis, prompting product developers to keep concentrations low and spell out allergy warnings. Regulatory agencies consider it a moderate hazard, not on par with heavy solvents or hormonal disruptors, but careful label reading stays important for dermatology patients and workers in chemical plants. Disposal brings extra scrutiny, especially to prevent accidental release to waterways, where breakdown products could stress aquatic life.
Industry players keep scanning for safer and more sustainable alternatives, but resorcinol monoacetate’s mix of stability and mild antimicrobial action continues to carve out a niche. Green synthesis champions experiment with water-based and enzyme-driven acetylation, aiming for less solvent waste and energy use. Medical researchers look at hybrid molecules coupling the acetate ester with other skin-benefiting agents, and environmental specialists track its biodegradation in urban wastewater, keen to flag persistent byproducts. Pharma companies push for clearer guidelines on concentration limits in over-the-counter creams, while research groups in niche polymers and adhesives dig into untapped specialty uses, hoping to stay one step ahead of stricter environmental rules.
Resorcinol monoacetate pops up in places you might not expect. It’s a white, powdery substance that often lands on pharmacy shelves in acne creams, wart removers, and medicated ointments. I remember combing through ingredient lists as a teen, desperate to clear up breakouts before a class picture. That word—resorcinol—turned up in products that worked better than the flashy brands stacked across the aisle. Dermatologists know it, pharmacists grab bottles from the drawer behind the counter, but most folks just want the thing that calms their skin and shrinks bumps.
Small, stubborn skin problems add up to a big headache for plenty of people. Think back to a time when you developed a rough spot or a cluster of tiny white pimples. Doctors reach for resorcinol monoacetate at concentrations low enough to avoid harsh reactions but high enough to break apart those thick, gritty plugs in pores. It acts as a keratolytic agent, which means it helps remove dead skin cells from the top layer of skin.
This ingredient doesn’t hog the spotlight—other chemicals like benzoyl peroxide or salicylic acid get more attention. Resorcinol monoacetate teams up with sulfur in some over-the-counter creams because together, they attack the dead cells and the bacteria that cause clogged follicles. Warts and calluses fade when applied regularly, as the skin softens and peels away the unwanted bits.
Dealing with skin conditions like acne, eczema, or thick calluses doesn’t always require a trip to the doctor’s office. Products with resorcinol monoacetate offer people a way to manage problems at home without strong antibiotics or harsh peels. The U.S. Food and Drug Administration keeps watch over ingredients in non-prescription creams for good reason. Researchers and toxicologists have weighed in: low concentrations of resorcinol monoacetate—around 2%—are safe for topical use. Higher amounts could lead to skin irritation or absorption into the blood, so guidelines stay strict.
Overusing any topical skin agent causes trouble. Redness, flaking, or even allergic reactions sneak up if the product is applied too often. People with sensitive skin, kids, and pregnant women should talk with a pharmacist or doctor before starting something new. I’ve seen people treat a simple spot and wind up with raw patches from scrubbing or layering too many strong treatments. Healthy skin care habits—using the right amount, sticking to gentle cleansers, moisturizing—keep problems in check.
Companies and regulators could do more by offering clear labels and plain-language guides. Sometimes the tiny print and chemical terms leave people guessing about safe use. Public health experts work on easy-to-read pamphlets and digital tools to offer better guidance, especially for those dealing with chronic skin troubles. Education makes a difference, whether it’s a pharmacist double-checking with a customer or a new parent looking up tips online.
Trustworthy information and safe products cover half the battle in health care. As someone who's seen the impact of skin problems on confidence and comfort, I notice the value in these tried-and-true remedies. Science supports resorcinol monoacetate’s use at the right concentration. Users just want relief from bothersome skin flare-ups, and the best results come by using well-researched, regulated solutions. That’s enough reason for me to keep an eye on how these ingredients evolve and how education reaches everyone who could benefit.
Skepticism tends to rise each time a new skin-care ingredient pops up on a label. People look for straight answers hiding among complex-sounding chemicals. Resorcinol monoacetate, sometimes found in medicated creams, isn’t a household name. Folks often wonder if applying it is a gamble or a benefit.
Resorcinol monoacetate works as a mild antiseptic and peeling agent. Chemists tag it in the same family as basic resorcinol, which already appears in some acne and wart treatments. Dermatologists have seen certain forms clear up rough spots, unclog pores, and peel away stubborn flakes. The Food and Drug Administration (FDA) has weighed in on both the risks and rewards of its cousin, resorcinol, on over-the-counter products by setting clear limits for safe use: concentrations up to 2% in topical drugs. Research on resorcinol monoacetate stays more limited. That lack of abundant data leads many professionals to rely on related studies to gauge potential.
Every day, people chase clear skin, willing to try the next trending ingredient. Still, plenty end up consulting dermatologists due to burning, redness, and irritation—especially those of us with sensitive or broken skin. Chemical exfoliants, even ones classified as mild, can tip the balance. I’ve seen firsthand how some well-meaning creams left raw patches because too much product lingered on my skin. Guidance from trusted skin doctors beats trial and error.
Peer-reviewed studies point to both the promise and the risks. Allergic reactions pop up in some users—itchiness, swelling, and rash don’t always wait long to announce themselves. Contact dermatitis isn’t uncommon, especially for those using more than prescribed or layering multiple active ingredients. Discoloration, or brown spots, can happen over time, particularly in people with darker skin tones. Conditions worsen if someone ignores sun protection or keeps slathering it on already irritated skin.
Resorcinol compounds, if overused or absorbed in high amounts, can affect the thyroid and blood cells. While most folks skimming on-the-shelf concentrations won’t run into these problems, these signals push health experts to advise sticking to small areas and short-term use.
Labels on skin-care bottles can feel like a foreign language. Skipping the ingredient list rarely leads to happy endings—especially for those with allergies, eczema, or open wounds. Listen to your body. If burning or swelling occurs, don’t tough it out. Flush the area, stop using the product, and see a doctor.
If resorcinol monoacetate appears in any new treatment, ask your dermatologist before giving it a trial run. People with thyroid issues, blood disorders, or already sensitive skin should steer toward extra caution. Testing a dab on one patch first helps root out possible reactions.
With so many products on shelves, safer, proven ingredients suit most routines—think moisturizers with ceramides or treatments with low-concentration salicylic acid. Skin safety rarely comes from shortcuts. My own experiments with harsh actives almost always meant running back to basics. Skilled professionals and evidence-based research guide the best decisions. Looking deeper than the marketing buzz makes room for lasting, healthy skin.
People reach for topical solutions hoping to clear up stubborn skin problems, but nothing comes free in the world of dermatology. Resorcinol monoacetate, often used in creams for skin lightening and acne treatments, brings both promise and risk. Many have dealt with uncomfortable tingling or stinging shortly after application. This isn’t just some minor inconvenience—redness, peeling, and swelling tell the story of a chemical that doesn’t always play nice with sensitive skin. Those with eczema or chronic dryness face a tougher road, sometimes finding their symptoms only get worse.
A few rare cases land people in the emergency room rather than a dermatologist’s office. Facial swelling or hives shouldn’t be brushed off as normal irritation. People who notice these signals may be experiencing an allergy to the compound. Shortness of breath or tightness in the throat spell out an immediate need for medical help; talking with an allergist after a reaction is critical.
It’s easy to forget that skin can absorb more than we think. Slathering on too much or using it on large sections, especially if there are open wounds, raises risk. Too much resorcinol can mess with thyroid hormones, leading to symptoms like tiredness, weight changes, or feeling cold all the time. Resorcinol acts by interfering with enzyme activity in the thyroid; I know someone who experienced weeks of feeling sluggish before realizing a simple skin cream was the culprit. Diagnosing thyroid issues that spring from a skin product can throw off even seasoned physicians.
Accidents happen and sometimes creams don’t stick to just the intended spots. Rubbing treated areas and then touching the eyes burns like wild and can damage the surface. If any gets in the mouth, it tastes bitter and can cause sores inside the mouth or around the lips. The warning label might not shout it, but this is more than a mild nuisance for people who already have sensitivities.
People often underestimate the impact of long-term use. I once thought applying over-the-counter treatments was harmless if used for only a few weeks, but that time adds up. Some users see skin discoloration or brownish patches after months of using resorcinol creams. There are recorded reports in medical literature documenting blue or gray skin tints, particularly in people with darker skin, which can quietly knock down self-confidence and spark questions about the safety of such ingredients.
Doctors recommend starting with small amounts, especially for those with past sensitivities. Reading ingredient labels sounds tedious, but it’s a small step for peace of mind. I always patch test on my own arm before fully trusting a product; a single night of discomfort beats weeks of regret. People with thyroid issues or a history of allergies need to listen closely to what their body tells them after application. Real progress in skincare means trusting real evidence and not letting a cream control the outcome.
Safer options often exist. Dermatologists can help steer folks toward newer treatments that skip harsh chemicals like resorcinol monoacetate. Investing in regular check-ins and keeping an open line with a healthcare provider goes further than hoping a product will perform miracles. Skincare works best as a team effort between patient, product, and practitioner.
Walking into any chemical storage room brings back that sharp memory of a time someone left a bottle of an organic powder too close to a sunlit window on a summer afternoon. It clumped, shifted color, and smelled different by the week’s end. If that powder happened to be something like resorcinol monoacetate, it’s not just a lesson; it’s a warning with real impact on safety and effectiveness. Keeping this chemical in top shape becomes less about following rules and more about paying attention to the details of your own environment.
Resorcinol monoacetate has that bland, powdery appearance, but don’t trust its looks. Moisture in the air catches hold of this compound quickly, turning a clean sample into a sticky headache. Dry places matter most. A good habit: check humidity in storage rooms with a digital hygrometer. Anything above 50% spells trouble for long-term shelf life. Relying on regular air conditioning often won’t cut it. For better protection, a sealed cabinet with a couple of fresh silica gel packets keeps moisture at bay.
Rustling around in labs over the years, I’ve seen people use old refrigerators for “cool, dry storage.” It only takes one leaky gasket to ruin a pricey batch overnight. For resorcinol monoacetate, aim for a stable, room temperature spot—away from direct heat sources and sunlight. Consistent mistakes with heat lead to slow but permanent degradation, even before the chemical gives obvious signs.
Glass bottles seem sturdy until you think about UV rays. Even those brown-amber bottles only block so much. Set containers of resorcinol monoacetate far from sunlit shelves and any fluorescent lamp that stays on all day. In spots where natural light creeps in, use opaque containers or slip bottles inside a simple cardboard box.
Some labs keep their most sensitive reagents in vacuum-sealed bags, but for day-to-day work, closing the jar tightly each time and storing in a low-traffic area gets the job done. If your workplace deals with questionable airflow—maybe near an open fume hood—expect contamination to sneak in. Even air with a bit too much dust can turn small containers of chemicals less pure.
Mislabeling seems impossible until devices change hands or months pass between uses. Use permanent markers with clear names and open dates. While resorcinol monoacetate doesn’t mix well with strong acids, oxidizers, or bases, store it on a separate shelf, never stacked together with those riskier compounds. Grouping it with similar neutral organic solids reduces confusion during a rushed day. Some workplaces set up weekly checks: a ten-minute walkthrough saves money and prevents mistakes by catching cracked lids, faded labels, or unexpected spills early.
Handling this compound always means gloves and a dust mask. Even clean air can carry a fine powder deep into the lungs. Wash hands before and after use. Small spills should get cleaned with a damp disposable towel rather than a broom, which only gets dust airborne.
Most advice on resorcinol monoacetate storage gets lost in technical jargon. People rarely mention the trouble caused by ignoring ordinary, everyday issues in storage areas—humidity, sunlight, airflow, and lazy labeling. Using this chemical with respect and vigilance helps avoid waste, health risks, and expensive replacements. The details matter most in the storage room, not just in the handbook.
Resorcinol monoacetate comes up for people who deal with skin issues. This ingredient often pops up in products for treating acne, warts, calluses, and sometimes even pesky cold sores. In my student days, I remember friends turning to over-the-counter lotions full of resorcinol when acne hit hard. With antibacterial properties, it helps break down thick or scaly skin, making it a useful ingredient for anyone bothered by stubborn bumps or rough patches.
Unlike some active ingredients in dermatology, resorcinol monoacetate does not line pharmacy shelves in its purest form. The pure compound rarely gets sold straight to the public due to respiratory and skin sensitivity concerns. The U.S. Food and Drug Administration (FDA) has flagged high daily uses of resorcinol as something to approach with care. Products using resorcinol monoacetate—think acne ointments, medicated creams, or wart removers—sometimes sit behind the pharmacy counter, or they join the over-the-counter lineup at established retailers, depending on the strength and blend.
Drugstores may stock low-strength cleansers or spot treatments containing related resorcinol or salicylic acid for mild issues. For more concentrated mixes or use on larger areas, a doctor weighs in, often writing a prescription for the right strength and directions. I’ve watched people walk in hopeful at pharmacies, only to learn their specific need demands a check-in with a dermatologist. Products above 2% concentration tend to slip past over-the-counter regulations and require a doctor’s blessing.
The debate around access centers on safety risks. High doses or incorrect use of resorcinol monoacetate trigger skin irritation, thyroid disruptions, and, in rare cases, something more serious like methemoglobinemia, especially for children. Not everyone reads labels thoroughly. I met a parent once who assumed “medicated” meant “gentle” and found their child’s rash much worse within a day. This kind of mishap pushes regulators to keep tighter control over higher concentrations.
Shoppers face confusion, especially when looking up “resorcinol” or “resorcinol monoacetate” in health forums, only to spot conflicting answers. Much of this confusion comes from different countries having different rules, and from companies being vague about which molecule sits inside each tube. The FDA, Health Canada, and European agencies all treat this stuff with caution, but drug facts sheets and pharmacists remain valuable guides when questions come up.
Clear labeling and responsible marketing go a long way. Product designers owe it to buyers to spell out ingredient names and concentrations, avoiding that confusing fine print. Pharmacists act as a bridge, steering people toward safe options and flagging when a doctor’s visit makes sense. Public health campaigns add another safety net, teaching teens and adults about risks. I once worked a summer in a pharmacy where poster boards showed side-by-side photos of safe use versus overuse—visual warnings that stuck much better than technical jargon.
With any powerful skin treatment, asking a pharmacist or doctor for advice isn’t a sign of weakness—just smart self-care. Careful attention to the strength and safety instructions isn’t just for regulators; it keeps our medicine cabinets safe at home.
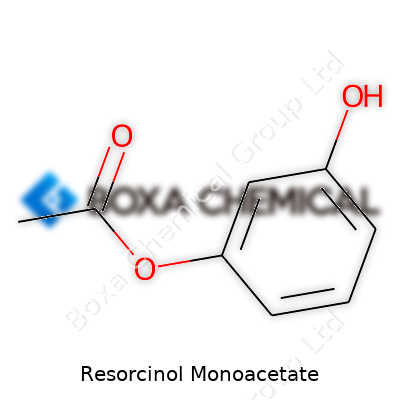
| Names | |
| Preferred IUPAC name | 4-acetyloxybenzene-1,3-diol |
| Other names |
3-Acetoxyresorcinol 2,4-Dihydroxyphenyl acetate RA Resorcinol monacetate |
| Pronunciation | /rɪˈzɔːrsɪˌnɒl ˌmɒnoʊˈæsɪteɪt/ |
| Identifiers | |
| CAS Number | [89-86-1] |
| 3D model (JSmol) | `6264.data.json` |
| Beilstein Reference | 585891 |
| ChEBI | CHEBI:9184 |
| ChEMBL | CHEMBL141324 |
| ChemSpider | 20733 |
| DrugBank | DB14051 |
| ECHA InfoCard | 100.007.784 |
| EC Number | 205-258-7 |
| Gmelin Reference | GN122480 |
| KEGG | C14377 |
| MeSH | D012101 |
| PubChem CID | 7379 |
| RTECS number | DH7525000 |
| UNII | NBG3AH98J9 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID5020746 |
| Properties | |
| Chemical formula | C9H10O3 |
| Molar mass | 196.19 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | odorless |
| Density | 1.28 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.88 |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | 14.42 |
| Magnetic susceptibility (χ) | -58.5e-6 cm³/mol |
| Refractive index (nD) | 1.571 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 151.6 J‧mol⁻¹‧K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -669.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2183.5 kJ/mol |
| Pharmacology | |
| ATC code | D11AX07 |
| Hazards | |
| Main hazards | Causes skin and eye irritation. Harmful if swallowed. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H302, H315, H319 |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 118°C (244°F) |
| Autoignition temperature | 132 °C (270 °F; 405 K) |
| Lethal dose or concentration | LD50 oral (rat): 3900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 3900 mg/kg |
| NIOSH | DF4550000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1-10% |