Curiosity about the chemical bounty within roots and bark drove early chemists to explore natural phenolics, with protocatechualdehyde standing out from the start. Isolation from natural sources like Salvia miltiorrhiza and vanilla pods forms part of a broader effort since the 1800s to decode plant defense mechanisms and flavor pathways. These botanists and early pharmacologists realized that this compound didn’t just build flavors—it carried medicinal punch. Over time, advances in extraction and synthetic chemistry broadened access. The road from mortar-and-pestle tinctures to today’s analytical chromatography reflects deeper trust in science and a hunger for reliable quality and standards. Generations of hands-on work laid the foundation, giving us modern insights but also hard lessons about the limits of plant-based extractions and the drive toward precision synthesis.
Protocatechualdehyde draws interest for its role as a versatile building block. The core structure—a benzene ring with two hydroxyls and an aldehyde group—shows up across pharmaceutical research, food science, and specialty chemicals. Manufacturers target a range of purities, shapes, and packaging. The most common form appears as light yellow crystals, quickly brownish when exposed to air, with a faintly spicy scent that hints at its plant roots. Using analytical controls like HPLC and NMR, producers vouch for identity, low impurity levels, and stable batch-to-batch characteristics. Clear labeling and regulatory compliance keep it out of gray-market shadows and ready for regulated labs and factories. Reliable supply and deep product data arm formulators and researchers with the confidence to explore creative applications.
This compound does not just sit quietly on a shelf. With a molecular formula of C7H6O3 and a molar mass of 138.12 g/mol, it melts around 137-140°C, boiling at higher temperatures depending on pressure. Those two phenolic OH groups influence solubility, skewing toward water and polar organic solvents like ethanol. The aldehyde group at the 3-position opens doors for reductions and addition reactions while making it prone to oxidation on contact with air. Under basic or acidic conditions, it can behave unpredictably, making careful handling essential during both storage and experimentation. Infrared and UV-Vis spectra show clear, sharp signals useful for purity checks, while strong reducing power often shapes its chemical reactivity, both in research and industrial contexts.
Commercial producers address a wide swath of users by listing purity as a headline spec, often not less than 98% by HPLC, with strict caps on heavy metals and moisture. Packaging tends to involve amber bottles with nitrogen purging for long-term stability, staving off the browning and loss of potency caused by oxygen and light. Labels give CAS numbers (139-85-5), batch codes, date of manufacture, and safety data, reflecting regulatory pressure and user demand for transparency. These technical claims hold weight for pharmaceutical quality audits, where data integrity and traceability carry legal and ethical clout.
Traditionalists pull protocatechualdehyde from plant sources using water or ethanol extractions followed by acid hydrolysis, then purify it through column chromatography or crystallization. Synthetic routes offer cleaner, more consistent product: oxidative hydrolysis of vanillin with agents like potassium permanganate or silver oxide creates a reliable pathway. Some labs adopt green chemistry, stirring up oxidation reactions with mild reagents in aqueous media, driving up yield and safety. Continuous improvements in extraction and purification shore up sustainability and cost control, with plant breeding efforts yielding root crops with naturally higher concentrations. Cleanup strategies after synthesis—filtering, recrystallizing, neutralizing byproducts—make the difference between high-confidence, research-grade product and an unreliable batch that might stall a month’s worth of experiments.
The distinctive aldehyde and phenolic groups create a launching pad for further synthesis. Reductions convert the aldehyde moiety to alcohols—useful for downstream pharmaceuticals with gentler bioactivity. Condensation with amines or thiols leads to Schiff bases or thioacetals, which show promise as enzyme inhibitors or antimicrobial agents. Sulfation, methylation, or glucosylation draw from nature’s own toolkit, boosting water solubility or biological stability. In the lab, coupling with other aromatic compounds produces complex polyphenols and ligands for catalysis. Each reaction serves as a proof-of-concept for new molecular scaffolds, often echoing the ways plants use protocatechualdehyde to fight off pathogens or build pigments.
This molecule answers to many names. Among chemists, the terms 3,4-dihydroxybenzaldehyde and protocatechuic aldehyde show up regularly. Some suppliers brand it simply by CAS number; others link to classic plant names like “Danshensu aldehyde” or legacy terminology from classical pharmacognosy. For regulatory filings and chemical trade catalogs, sticking to IUPAC names avoids mix-ups with related phenolic aldehydes. Online and in international commerce, product cross-referencing reduces shipping surprises and meets customs requirements.
Despite a background linked to edible plants, protocatechualdehyde requires a careful hand. Dust and vapors irritate airways, and the reactive aldehyde group raises the specter of skin sensitization. Handling calls for chemical-resistant gloves, splash protection for eyes, and access to fume hoods. Data sheets set exposure limits and describe symptoms spanning redness to breathing discomfort after heavy exposures. Fire safety matters too: dry form can feed combustion alongside organic solvents. Storage away from bases and oxidizers, in tightly capped glass, keeps accidents and degradation at bay. Waste disposal leans on regulated incineration or chemical neutralization, backing up both environmental and occupational safety.
Protocatechualdehyde has built a reputation as more than just a plant curiosity. Its antioxidant activity spurs nutraceutical formulations aimed at heart, liver, and skin health. Early Chinese medical texts describe herbal concoctions rich in this moiety for improving circulation and fighting “blood stasis syndrome,” findings echoed by clinical research. Pharmaceutical chemists see useful anti-cancer, anti-inflammatory, neuroprotective effects, sparking patent claims and in vitro studies. Flavorists and food scientists use low-dose forms for gourmet products, while environmental chemists explore its radical scavenging to reduce food spoilage and extend shelf life. This diversity rests on a backbone of published studies and regulatory filings, where documented effects and regulatory status determine how widely companies can market finished products.
R&D teams keep stretching the limits. Universities and startups screen derivatized versions for better bioavailability or gentler metabolic handling. Animal models test anti-diabetic and liver-protective capabilities; data sometimes leap from mice to volunteers in early-phase trials. Materials scientists build thin films or polymers incorporating the compound’s redox activity, improving packaging or biosensor devices. Analytical chemistry journals feature new ways to detect, quantify, and track this molecule inside plant extracts or human plasma. The roadblocks—batch-to-batch variation, solubility shortfalls, metabolic instability—drive fresh waves of collaboration between organic synthesis and formulation experts.
Detailed lab work answers lingering questions about how safe this compound is at different doses and in various routes of exposure. Acute and sub-chronic toxicity studies in animals reveal that effects depend on administration pathway, dose, and animal species. Generally, the LD50 appears high—suggesting low acute toxicity by oral routes—but chronic dosing presents challenges. Some studies flag liver enzyme changes at high levels, especially for stress-prone rodent models, urging researchers and regulators to pin down safe limits for supplements and food use. Genotoxicity and mutagenicity panels give encouraging results—no prominent mutations seen in bacterial cultures or mammalian cell lines—yet reproductive and developmental toxicity deserve more clarity, especially with consumer-facing health products.
This compound’s journey is far from over. Modern medicine’s pivot toward plant-based molecules and precision nutraceuticals matches up with protocatechualdehyde’s broad pharmacological profile. Supply chains shift toward greener synthesis and crop engineering to answer rising demand. On the therapeutic front, tailored analogs and prodrugs may clear regulatory hurdles, offering better targeting for diseases like ALS, Alzheimer’s, or rheumatoid arthritis. The food sector may see more antioxidant-rich packaging and clean-label additives. The synthesis in smart materials—from catalysts to UV-absorbent polymers—adds another revenue stream. Investment in comprehensive toxicity documentation and responsible sourcing will shape access in North America, Europe, and Asia. Success will favor those combining solid scientific research, manufacturing transparency, and ethical stewardship of natural resources.
Every so often, science uncovers something in nature that’s more useful than it looks. Protocatechualdehyde stands out as one of those cases. This plant-based molecule shows up in foods like olives, grapes, and certain grains, but most people walk past it on every nutrition label without knowing what it does. Scientists and doctors, on the other hand, pull their chairs closer when they see research about this compound.
I’ve noticed people sometimes glaze over when they hear chemical names. That feeling disappears when they start seeing direct health impacts. Protocatechualdehyde has drawn attention for its strong antioxidant activity. In my own search for healthy living, antioxidants matter; they fight free radicals, which contribute to aging and cell damage. Research from years past, including studies published in journals like “Food and Chemical Toxicology,” documents how this molecule can help tackle inflammation and lower oxidative stress. That benefit alone puts it on the radar for treating chronic problems—from heart disease to some types of brain conditions.
Medical science often focuses on drugs for treating illnesses, but natural compounds often lay the groundwork for bigger discoveries. Researchers have been testing protocatechualdehyde for its impact on cancer cells. I remember reading a study from 2019 showing how it slowed the growth of certain cancer cells in the lab. While no one suggests swapping a doctor’s prescription for it, this type of data pushes the search for new treatments forward.
Traditional medicine uses substances like this long before labs analyze them. Chinese medicine, for example, uses protocatechualdehyde-rich herbs for everything from cardiovascular support to fighting infections. Modern science backs some of these uses up, with evidence its anti-inflammatory qualities could help manage things like arthritis as well as some infections. My skepticism dropped once I saw consistent results, both in clinical trials and in conversations with people who used herbal remedies as part of their health routines.
What makes this molecule valuable isn’t just health. It pops up in the food industry because it resists spoilage—clues suggest it could help foods last longer or stay fresh. As someone who values reducing waste, that matters to me. On the technical side, chemists have used it for making new materials and dyes, taking advantage of its reactivity. These all offer economic opportunities, especially for agribusinesses looking to use every part of a harvest.
Still, the picture isn’t perfect. Large-scale production can cost a lot. Consistent dosing—crucial for medical use—remains difficult. Regulatory bodies want deep proof of safety. In my experience, many natural compounds stall at this stage. Good research, quality control, and regulatory openness can move things forward, but this takes funding and patience.
Protocatechualdehyde’s story isn’t finished. In my view, it offers more promise than hype—but only if researchers stay focused and honest about both benefits and risks. Food scientists, doctors, and chemists should keep working together so more people can benefit from what nature quietly offers. With better funding for applied research and stronger connections between traditional and modern knowledge, this compound could make a real difference in medicine and industry alike.
Protocatechualdehyde shows up in some products you wouldn’t expect. Researchers have spotted it in plants like barley, onions, and even the bark of some traditional medicinal herbs. Its chemical structure—part phenolic aldehyde, part antioxidant—grabs the attention of scientists poking around for natural compounds that could help human health. But with so much buzz online about what’s “natural,” people deserve to know whether it’s actually safe to consume or touch this substance.
The first thing most folks want to know is whether protocatechualdehyde feels toxic. Studies on rats and mice help sketch an early picture, even if animals don’t always react like humans. Doses given to rodents rarely stir up reports of major harm. Organs seem to function as normal and immune systems don’t freak out. That said, most of this testing focuses on short-term use and limited quantities. Big doses delivered over a long stretch? Most human trials haven’t gone that far, so nobody can call it bulletproof.
Some health blogs refer to protocatechualdehyde’s “anti-cancer” or “anti-inflammatory” qualities. Digging into published research, the compound does block some oxidative damage and even slows the growth of certain cancer cells in Petri dishes. But test tubes and petri dishes live a different reality than the human body, which juggles countless variables. A little bit of hope exists, although jumping from early lab wins to broad proclamations of safety skips over important gaps.
People who cook at home likely eat trace amounts already. Whole grains, olive oil, and some teas contain low levels naturally. Nobody has recorded a rash of allergic reactions or illnesses from these foods, which tells us that tiny exposures don’t usually stir up trouble. This matches up with a larger pattern: humans, through thousands of years of eating plants, generally handle plant phenolics like protocatechualdehyde without outlandish side effects. Big differences emerge with supplements or extracts, where concentrations skyrocket way beyond what you’d munch in a salad.
Regulatory bodies—like the US Food and Drug Administration or the European Food Safety Authority—set ground rules for supplement makers and researchers experimenting with natural compounds. So far, neither group has handed out a rubber-stamped approval for protocatechualdehyde as a food additive or a nutrition supplement. That means there’s no guarantee of purity, dosage, or even a broad consensus about what happens with long-term exposure. Well-established safety testing just hasn’t caught up yet.
As an ordinary person following the debates, I care about what goes into my body, whether it’s a fancy new ingredient or a time-honored one. Reading ingredient lists and picking food in unprocessed forms works for my family, especially since it’s hard to trust what’s in powders and capsules shipped from unknown sources. Playing it safe beats jumping on hype. If you’re considering new supplements, the advice from my own doctor focuses on moderation, plus checking for allergies or interactions with medicine.
The story with protocatechualdehyde echoes lots of other “emerging” health compounds: long tradition doesn’t always equal proven safety at high doses. Waiting for better evidence and smarter regulations keeps things safer for everyone.
Protocatechualdehyde shows up as a light yellow crystalline solid—quiet in appearance but not in significance. If you’ve spent much time working with organic compounds in a lab, you’ll notice that it dissolves quite nicely in water and most alcohols. This solubility makes it very approachable if you’re trying to use it in research or scaled-up processes. Its melting point hovers around 153 to 157°C, which tells you it’s stable enough for a lot of applications but not so stubborn that you can’t melt it without special equipment. The smell stands out—sharp, distinct, much like a hint of benzaldehyde mixed with a touch of earthiness. You’re not forgetting that aroma if you’ve handled it.
Handling protocatechualdehyde feels straightforward if you’re used to working with phenolic compounds. It doesn’t readily sublimate or evaporate at room temperature, which means storage is not a headache. You keep it in a tightly sealed container, dry and away from light, and it sits there just fine.
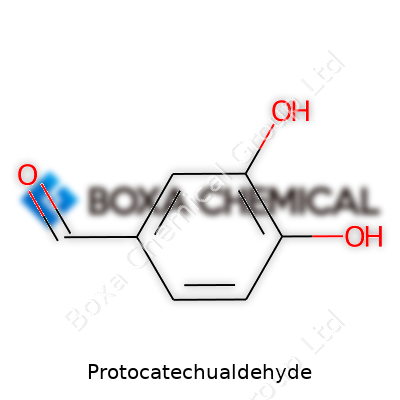
This compound stands on two catechol-like hydroxyl groups positioned on a benzene ring, with an extra aldehyde group at the third carbon. This small addition makes a huge difference. Unlike simple catechol, that aldehyde group gives it a reactive edge, nudging it into a different category for pharmaceutical and chemical research. Its formula—C7H6O3—may not sound exciting, but the molecule brings interesting chemistry due to the balance between its polar functional groups and aromatic backbone.
From a chemical reactivity point of view, you get classic phenol-type behavior along with the ability to undergo condensation and redox reactions thanks to the aldehyde. You put it in a basic environment and those hydroxyl groups show their usual activity, forming phenolate ions that drive some powerful reactions. Add in strong oxidizers and you see the aldehyde group go to work, oxidizing to carboxylic acids. These features allow scientists to use protocatechualdehyde as a versatile building block in synthesis or as an intermediate in various bioactive compounds.
My experience in the lab brings a lot of respect for this molecule’s dual role—both as a research tool and as an actor in traditional medicines. Some clinical studies highlight its antioxidant and anti-inflammatory properties. This only works because of the chemistry: those reacting hydroxyl and aldehyde groups let it scavenge free radicals efficiently. Research communities keep turning to it for these reasons, especially as interest grows in plant-derived compounds.
Still, nothing’s perfect. The reactive groups mean it’s not completely stable if left exposed. You need proper conditions to keep it from polymerizing or degrading. That underscores the importance of careful handling—something lab safety protocols have taught me through hard-earned experience. Keeping it away from strong oxidizers, acids, or bases unless intended is vital. Missteps lead to product loss and safety risks, so it pays to take your time and set the stage properly for experimentation.
To take full advantage of protocatechualdehyde, modern research and industry could do more to improve storage and transportation methods. Improved packaging to limit light and moisture exposure would extend shelf life and maintain its properties. More studies can also reveal new therapeutic uses, especially given the ongoing search for low-toxicity, plant-derived antioxidants. Researchers would do well to share negative as well as positive results to speed up understanding and safe application. This approach draws from years of seeing potential missteps firsthand and knowing that robust, real-world science happens when no corner is cut.
Protocatechualdehyde may not get as much attention as larger or flashier molecules, but it does the work, quietly solving problems that cut across chemistry, biology, and medicine.
Anyone who's handled sensitive chemicals in a lab knows how much a small mistake can cost. Protocatechualdehyde comes with its own set of quirks. At first glance, it seems like a regular powder or crystallized substance, but it packs a punch if left unchecked. Unlike some household chemicals, this one reacts to air and light. Leaving it exposed for even a short period can set off unwanted oxidation, turning your carefully weighed sample into something brown and unreliable.
From personal experience, it’s never fun watching a reagent degrade just because someone left the lid loose. Choose amber glass bottles, since regular plastic doesn't cut it — nothing ruins an experiment like leaching or cross-contamination. Tightly sealed containers offer the simplest shield against unwanted moisture and air, which can kick-start unwanted reactions or ruin your next batch. In my early research days, I saw more than one project delayed just because someone tried to cut corners with cheap storage.
A lot of labs run warm, especially in the summer, and that’s where storage goes from boring to make-or-break. Protocatechualdehyde needs a cool, dry place, ideally below room temperature. Direct sunlight isn’t just bad for plants in a lab window; it will break down this compound as well. Use the chemical fridge or storage cooler, away from acids and bases. Mixing storage with highly reactive agents makes about as much sense as storing milk next to open fish in the fridge: something will go sour fast.
Humidity stands out as a silent threat. Silica gel packs are simple to order and cheap. Toss a couple in every storage bottle. During one humid spring in the lab, we lost an entire supply of not just protocatechualdehyde but three other phenolic compounds. A $2 pack of desiccant felt like a missed investment.
Nobody wants a trip to the emergency room. Gloves, goggles, and a standard lab coat might feel repetitive, but skin contact with this aldehyde can cause serious irritation. Small spills should get cleared up with proper absorbents, not just wiped with a lab paper towel. Always check the chemical’s safety data sheet before lifting the cap, even if you think you remember the drill.
Working under a fume hood is about more than just preventing chemical odors — it can be a lifesaver. Even brief inhalation of certain aldehydes leaves you with a burning throat or eyes for the rest of the day. While direct exposure might not cause immediate disaster, repeated carelessness chips away at your health. Keeping a tidy workspace, labeling every bottle with the date, and logging usage makes a huge difference.
Experience and training always show their value during hectic days. When every member of the team understands proper storage and handling—down to always closing the cap and updating the inventory—costly mishaps don’t happen nearly as often. Supervisors who take the time to run demos and refreshers leave fewer chances for someone to learn things the hard way.
Reports estimate that improper storage and handling cause about 25% of all lab accidents. Investing a small amount of time in building good habits creates safer, more productive spaces. If somebody sees a fellow researcher about to store aldehydes next to incompatible chemicals, it’s better to speak up right away than patch things up later. There's no shortcut to safety when it comes to chemicals with real risks attached.
I’ve been around research labs long enough to recognize the distinct smell of a chemical bottle fresh from a supplier. Protocatechualdehyde isn’t a name you hear over coffee, but anyone who has worked with plant research, synthesis, or advanced chemical projects probably knows it by sight, at least on a label in the fridge. Often, the practical challenge does not come from understanding a molecule’s potential, but from the simple act of purchasing it without unnecessary hassle or risk.
Trustworthy chemical procurement anchors itself to reliable sources. Sigma-Aldrich, Thermo Fisher Scientific, Tokyo Chemical Industry (TCI), and Santa Cruz Biotechnology still hold strong positions among global suppliers for researchers needing protocatechualdehyde. Most offer detailed certificates of analysis and safety data, which help lower the risk of contamination or poor quality. These established suppliers also require end-use declarations or institutional accounts, which makes sense. Their products sometimes end up being precursors for more sensitive discoveries, so they avoid letting anything slip through to unauthorized buyers.
As a scientist outside industry, I’ve run up against red tape trying to order something as basic as protocatechualdehyde. You run into questions about your institution, the nature of your research, and proof that your facility handles chemicals with proper oversight. Businesses selling to the general public stay away from most regulated chemicals, steering clear of legal headaches and shipping nightmares. For independent projects, especially outside academic or industrial labs, options shrink fast.
Buying directly from one of the big names usually means having a verified commercial or academic account. E-commerce sites like Alibaba and ChemSpider list international sellers, but the risk of counterfeit or impure product hovers over those sources. Even scanning through chemical forums, I see plenty of warnings about unknown suppliers. Regulations in the US, EU, India, and China call for honest documentation, but smaller vendors sometimes cut corners on batch tracking or export rules. It pays to check certifications and look up reviews—angry researchers don’t stay quiet online for long.
The price for protocatechualdehyde can be a shock. Expect single grams to run anywhere from $60 to $150 from market leaders, with discounts sometimes available for larger orders. Quality control and shipping add costs, and small-quantity buyers rarely get a break. Bulk wholesale, straight to a research center, sometimes drops the price per gram to $20 or less. Factor in customs fees, taxes, and storage expenses; the sticker price online is just the start.
It’s rare to find genuine product below market rates from a reputable source. I’ve seen deals on less-transparent sites, but nothing ever convinced me to stray from trusted suppliers. Academic collaborations sometimes help researchers pool orders and save on shipping. For small operations, this team approach doesn’t just cut costs, it builds community—something that helps when sourcing rare chemicals becomes a group challenge.
Transparent pricing, responsible sourcing, and proper documentation matter just as much as the molecule itself. Protocatechualdehyde offers plenty in the worlds of medicine and diagnostics; getting it safely into the hands of skilled researchers depends on strong trust up the supply chain. In my own experience, investing the time to check supplier credentials, network with experienced scientists, and collect every bit of paperwork, avoids risks that can cost more than money. The safest, most efficient path usually runs through an established supplier, even if that means spending a little more up front.
| Names | |
| Preferred IUPAC name | 3,4-dihydroxybenzaldehyde |
| Other names |
3,4-Dihydroxybenzaldehyde PCA Protocatechuic aldehyde 3,4-Dihydroxybenzal 3,4-DHBAL |
| Pronunciation | /ˌproʊ.tə.kəˌtɛk.jʊˈæl.də.haɪd/ |
| Identifiers | |
| CAS Number | 139-85-5 |
| Beilstein Reference | 1362432 |
| ChEBI | CHEBI:16906 |
| ChEMBL | CHEMBL1136 |
| ChemSpider | 65143 |
| DrugBank | DB04241 |
| ECHA InfoCard | 100.027.975 |
| EC Number | 1.2.1.53 |
| Gmelin Reference | 724213 |
| KEGG | C00633 |
| MeSH | D011434 |
| PubChem CID | 869 |
| RTECS number | UY8750000 |
| UNII | U9QD207GXW |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C7H6O3 |
| Molar mass | 138.12 g/mol |
| Appearance | Light yellow needle-like crystals |
| Odor | aromatic |
| Density | 1.129 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.05 |
| Vapor pressure | 0.00156 mmHg (25°C) |
| Acidity (pKa) | 7.40 |
| Basicity (pKb) | 13.52 |
| Magnetic susceptibility (χ) | -64.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.618 |
| Viscosity | 1.197 mPa·s (25 °C) |
| Dipole moment | 2.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 153.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -211.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1434.9 kJ/mol |
| Pharmacology | |
| ATC code | A15AX18 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 110°C |
| Autoignition temperature | 522 °C (lit.) |
| Explosive limits | Explosive limits: 2.4–14% |
| Lethal dose or concentration | LD50 (rat, oral): 2220 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2220 mg/kg |
| NIOSH | NA3430000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/kg bw |
| Related compounds | |
| Related compounds |
3,4-Dihydroxybenzaldehyde Vanillin Protocatechuic acid Catechol Syringaldehyde |