2,4,6-Trimethylphenol entered the chemical world during an era that saw rapid expansion in aromatic compound research. The substance caught attention among chemists pushing for alternatives to naturally derived phenols, as industries searched for ways to improve yields and reproducibility. During the 20th century, once synthetic routes matured and large-scale phenol alkylation became cost-effective, this compound found solid ground in the laboratory, setting a precedent for using substituted phenols in research and manufacturing. My university lab, for example, used different alkylated phenols as model substrates, so 2,4,6-Trimethylphenol never felt exotic—its story is bound to the larger drive to create predictable phenolic building blocks.
2,4,6-Trimethylphenol presents as a crystalline solid with a slight phenolic odor. On close handling, the substance leaves a gentle, distinct scent on gloves and glassware—a feature many researchers recall. As a derivative of phenol, it stands out due to its three methyl groups attached to the aromatic ring, which shape both its reactivity and niche uses across labs. Chemists lean into its methylation pattern to guide downstream reactions, particularly when exploring new synthetic pathways for antioxidants or specialty additives.
The compound melts near 44°C, noticeably lower than many other phenol analogues, and boils around 230°C under atmospheric pressure. Lab work with 2,4,6-Trimethylphenol shows its moderate volatility and solid nature at room temperature, making for consistent handling. Its poor solubility in water challenges quick cleanups but helps with certain crystallization protocols. With a molecular formula of C9H12O, the density lands close to 0.96 g/cm³ in liquid state. The methyl groups buffer the phenolic hydrogen, making the compound less acidic compared to phenol—a detail that reveals itself during titration.
Suppliers often provide 2,4,6-Trimethylphenol at purities above 98%. Researchers need clear labeling due to its similarity to other trimethylphenol isomers. The product arrives with batch numbers, purity percentages determined by GC or HPLC, and recommendations for storage in cool, ventilated spaces. Because regulations tighten around the handling of phenolic compounds, material safety data sheets include instructions on spill management, eye protection, and recommended use of gloves resistant to aromatic solvents.
Preparing 2,4,6-Trimethylphenol involves methylation of phenol using methylating agents—typically methyl chloride or dimethyl sulfate—over acidic or basic catalysts. Industrial sources favor Friedel-Crafts alkylation of phenol with tert-butyl groups followed by demethylation, which gives higher yields and cleaner product. Researchers sometimes engage in direct methyl group introduction using sodium hydroxide and methyl iodide under phase transfer conditions, though this approach usually lags behind on efficiency metrics. These reactions demand careful temperature control to avoid over-alkylation and unwanted byproducts that can snarl purification efforts.
2,4,6-Trimethylphenol performs as a versatile aromatic substrate. The methyl groups at ortho and para positions block many electrophilic substitution routes, channeling reactivity to specific positions. This selectivity supports the synthesis of advanced ligands, antioxidants, and fine chemicals. For example, oxidative coupling yields complex biphenolic structures, and sulfonation—though challenged by the methyl shields—proceeds under rigorous conditions. The compound resists halogenation at blocked sites, and its phenolic oxygen opens up avenues for ether or ester formation.
People working with this substance encounter multiple names: mesitol, 2,4,6-trimethylhydroxybenzene, and 2,4,6-TMP. In catalogs and academic papers, the name “mesitol” often appears, while systematic nomenclature dominates technical sheets and regulatory filings. Synonym confusion sometimes triggers mix-ups, especially in multilingual environments, so professionals rely on CAS numbers to cut across naming conventions.
Lab experience with 2,4,6-Trimethylphenol underscores the need for robust safety protocols. The compound irritates skin, eyes, and respiratory systems. Airborne dust, albeit less common due to its low volatility, still poses risks in confined spaces. Best practice includes working in fume hoods, using goggles, nitrile or neoprene gloves, and having spill absorbers ready. Disposal channels lean on solvent-transfer waste streams rather than sink dilution to comply with environmental standards. Workers in manufacturing settings train specifically on emergency eyewash routes and the need for shower accessibility.
Industries involving antioxidants, specialty polymers, and UV-stabilizers lean heavily on 2,4,6-Trimethylphenol. Phenolic resins benefit from its methylated architecture, lending the finished materials increased stability and resistance to degradation. Paints and coatings tap into its ability to boost oxidative resistance, while pharmaceutical researchers examine its framework as a starting point for new synthetic drugs. Some agricultural formulations use methylated phenols to heighten shelf-life and preserve efficacy—though regulatory scrutiny is rising to keep tabs on long-term health impacts.
Chemists continue to reimagine how to harness 2,4,6-Trimethylphenol in complex molecule synthesis. University labs explore catalytic routes to relay methyl groups into the ring more selectively, cutting waste and reagent use. The search for greener methylating agents occupies a large space in grant-funded investigations, pairing the substance’s value with sustainability targets. Newer work examines how to integrate the compound into functionalized polymers and dendritic structures, which play roles in sensing, separation, or bioactive material design. Scientists publish findings about subtle tweaks to the methylation strategy that can cut down reaction times or lower the temperature needed for efficient alkylation.
Decades ago, safety data surrounding 2,4,6-Trimethylphenol remained thin, mostly informed by analog studies. Now, animal studies and in vitro work reveal moderate acute toxicity, displaying oral LD50 values in the gram-per-kilogram range for rodents. Eyes and skin remain particularly vulnerable, with repeated exposure amplifying irritation and risk of allergic response. Environmental studies detect that the compound slows breakdown in aquatic environments—so strict limits curb its release during manufacturing. Research now examines chronic effects, taking cues from broader phenol toxicity data to anticipate and manage occupational and ecological risks. Epidemiological data lags behind, so calls for more detailed, long-term population studies grow louder.
As industries hunt for alternatives to traditional phenolic stabilizers, demand for 2,4,6-Trimethylphenol looks set to climb. Progress in green chemistry will likely reshape how the compound gets made—both by shifting feedstocks away from petroleum sources and by finding catalysts that shrink the environmental footprint. Advances in polymer chemistry invite mesitol-based additives to serve a wider spectrum of plastics and elastomers. Researchers hope to unlock its potential in medicinal chemistry, designing derivatives with bioactive profiles to address inflammation or microbial threats. Companies moving into low-toxicity resins and coatings may chart the course for new regulatory frameworks centered on long-term ecosystem safety. Calls for more open data and collaboration across borders keep pushing the field forward, making this compound a touchstone for the next chapter in aromatic chemistry.
2,4,6-Trimethylphenol sounds like something reserved for industrial scientists, but its impact spills over into plenty of areas that reach right into daily life. This chemical sits among the family of methylphenols, straight out of the laboratory, yet its contributions chain out into different products and industries.
Manufacturers turn to 2,4,6-Trimethylphenol in the quest for tough, high-performance resins. These materials end up shaping important parts in electronics, automotive systems, and home construction. The molecular structure helps resin makers dial in properties like heat resistance and hardness, qualities you find in things like printed circuit boards and coated surfaces.
Years back, I watched technicians in a coatings lab sweat over the balance of durability with flexibility. 2,4,6-Trimethylphenol became part of their recipe for easily applied, long-lasting finishes used on wood and metal — making cabinets and machines last longer without losing their good looks.
Many specialty chemicals start out with building blocks, and 2,4,6-Trimethylphenol is a regular stop on that journey. Chemists turn it into antioxidants, stabilizers, and even additives for lubricants that push engines to run quieter and last longer.
Antioxidants based on this compound land not just in car engines but in hard-working machinery on farms or factory floors. Reliable antioxidants slow down rust, decomposition, and breakdown. In the long run, that saves money and prevents waste, since parts don’t fail as quickly.
Trace forms of 2,4,6-Trimethylphenol appear during the production of some flavor and fragrance compounds. The quantities stay low, but the chemical’s structure offers a starting point for more pleasant molecules. Skilled chemists transform it, taking advantage of its reactive spots. Without such intermediates, the path to the perfumes, soaps, and even food flavors most folks enjoy would come with more hurdles and higher costs.
Using chemicals safely can make or break trust in a company. 2,4,6-Trimethylphenol calls for careful handling — it's flammable and can irritate eyes or skin if spills aren't cleaned up fast. Producers handle this substance using proper ventilation, gloves, and storage away from heat. Over the years, I’ve seen that careful attention in industrial plants can head off accidents and keeps surrounding communities at ease.
Oversight groups like the EPA and OSHA keep tabs on how it’s moved, stored, and used within the U.S. That’s no nuisance; regulations protect both workers and the environment. Responsible companies don’t cut corners and keep up with worker education, regular inspections, and improved emergency planning to avoid fires or exposure.
More companies examine their supply chains these days, aiming to lower reliance on risky chemicals without losing quality. Scientists keep innovating, exploring alternatives or better ways to capture byproducts for recycling. Such steps can drive down waste and limit environmental risks linked to chemical manufacturing.
With the right approach, industries benefit from those foundational chemicals like 2,4,6-Trimethylphenol while pushing for safer, cleaner operations, not just for immediate profits, but to support a better future for everyone.
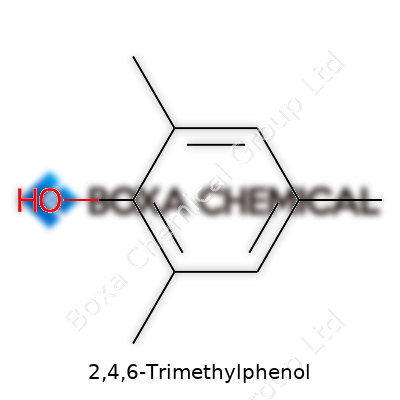
Walking through any college chemistry lab, you’ll find labels for all sorts of aromatic compounds. In the real world, 2,4,6-Trimethylphenol shows up as one of those niche molecules that helps bridge what students read in textbooks and what manufacturers use at scale. The chemical formula for 2,4,6-Trimethylphenol is C9H12O. It’s a simple formula, but the arrangement offers some interesting chemistry and relevance to industries far from the classroom.
A benzene ring sits at the core of 2,4,6-Trimethylphenol’s structure. Three methyl groups (–CH3) attach to carbons 2, 4, and 6 on the ring, leaving a hydroxyl group (–OH) at position 1. This isn’t some academic distinction—positions make all the difference once the molecule goes into a vat or starts reacting with other chemicals. It helps to picture how electron shuffling in aromatic rings can switch the properties of the product. As a student, I spent a whole afternoon sketching structures just to get that point, and the pattern stuck with me decades later.
Looking at where C9H12O ends up, one key area is the manufacture of certain resins and plastics. Without specific molecules like 2,4,6-Trimethylphenol, whole lines of thermoplastics wouldn’t have their signature strength or reliability. In another example, it fits into the puzzle of antioxidant production, which supports the stability of lubricants used in daily transportation and machinery. Products behind the scenes often shape reliability most people take for granted—think about engine oils resisting breakdown, or consumer goods lasting longer thanks to just the right additive.
Exposure and waste from industrial processing always need blunt conversations. Handling aromatic phenols involves clear risks—skin and respiratory irritation, environmental toxicity, and persistent waste streams. Too many cases show missing controls can lead to community exposure, runoff into water systems, and persistent residue. Effective containment, rigorous ventilation, and personal protective gear blunt those risks. Enforcement of protocols isn’t just a best-practice checklist—it directly protects workers and neighbors.
The search for safer versions and better controls keeps a tight circle between academic disciplines, regulators, and industry chemists. Substituting or designing routes that use fewer hazardous intermediates proves challenging, yet continual progress shows promise. My experience working with chemical engineers who constantly tweak process flows showed how minor adjustments—shifting a temperature, swapping a catalyst—can drop emissions or cut waste by a surprising margin. Lessons like that reinforce why training, strict oversight, and transparency hold real value for safety and innovation together.
Knowledge isn’t just about memorizing a formula like C9H12O. For chemists, environmental monitors, and anyone moving product from lab to plant, the details of structure and safety build a more robust system. Encouraging researchers and industry to invest in greener chemistry, smarter containment, and honest risk assessment pulls everyone forward. Each small step pays off, and focusing on those details delivers real outcomes—smarter products, safer workplaces, and a lighter environmental touch.
2,4,6-Trimethylphenol shows up as a white to yellowish solid. Factories use it for chemical synthesis, often as part of dyes, resins, and other industrial intermediates. You’ll find it mentioned on safety sheets, and researchers have looked at what it might do to the human body and the environment.
Many chemicals carry a certain reputation, but people want to know what happens if they touch, breathe in, or accidentally swallow a chemical like 2,4,6-Trimethylphenol. The most direct effects hit the skin, eyes, and respiratory system. Contact with skin can cause irritation. Breathing in a dust or vapor form may prompt coughing, burning in the nasal passages, sore throat, or headaches. Swallowing is not common, but if it happens, expect nausea or abdominal pain.
I’ve worked in labs that had a strict routine for storing this compound. Personal protective equipment was a must, not just to tick a liability box, but because small spills left unchecked often caused contact rashes and quick, sharp eye irritation. Such reactions line up with reports from the U.S. National Institutes of Health Hazardous Substances Data Bank. Even low-level exposure tends to bother sensitive skin or unfussy lungs.
Nobody likes to jump to panic, but animal studies do matter here. Rats exposed to high airborne levels of 2,4,6-Trimethylphenol showed changes in weight and some evidence of liver stress. That means the compound can build up in mammals if they breathe or absorb large amounts over time. Unlike known carcinogens, this chemical hasn’t set off alarms for cancer or DNA mutations as of 2024’s research. But toxicity does show up with repeated, high-level exposure.
The U.S. Environmental Protection Agency includes 2,4,6-Trimethylphenol on its hazardous substance lists. Usually, this means the agency watched or documented harm in test organisms, or in handling accidents at the industrial scale. The European Chemicals Agency scores it as irritating, not acutely fatal in tiny amounts, but needing careful handling. No surprise, then, that guidelines steer clear of drinking water pollution and breathing too much dust at work.
This compound doesn’t break down as fast as some solvents do, and sewer water can carry it downstream. That introduces risk for aquatic life and animals along the food chain. Runoff from factories that make or use it may show higher toxicity in fish and small invertebrates. Crew members working with the raw solid or its vapor need gloves, tight storage containers, and regular air checks. If the containers crack or spill, it takes a proper cleanup, not just a mop or paper towel thrown in the trash.
Better awareness and tough storage routines give workers and communities a real advantage in dealing with 2,4,6-Trimethylphenol. Plant operators install good ventilation and smart training, not just to avoid legal risk, but to dodge the headaches and eye burns that workers recall after exposure. Government agencies keep updating their exposure limits as more research fills in the gaps.
Local water systems should run regular checks downstream from plants, making sure the numbers stay below the EPA’s allowed thresholds. Workers can push for more skin protection and air monitors in areas where the chemical starts as a solid but might turn to vapor or dust during use. These real-world steps often have more bite than paperwork promises.
Some companies already turn to replacement compounds where possible, or push for closed-system handling to keep vapor and dust in check. Using experience—alongside new toxicity data—sharpens judgment. Most risks with 2,4,6-Trimethylphenol can be managed by paying close attention and sticking to strong workplace habits.
2,4,6-Trimethylphenol doesn’t ring a bell for most people, but in the chemical industry, proper storage sets the tone for everything else. A chemical with this structure stands out: volatile, flammable, and irritating. Personal experience tells me that attention to small details in storage stops bigger problems later on, both for people and for operations.
It sounds simple: Store it in a cool, dry spot. Yet, failing to control humidity or temperature in a warehouse can turn a manageable chemical into an accident waiting to happen. I remember walking into a facility in the summer—minimal ventilation, direct sunlight streaming on containers, and the sharp scent of phenol in the air. Those few degrees above room temperature not only push up the risk of fire, but long-term exposure encourages slow, silent degradation. Solid, labeled containers kept away from moisture and heat make a world of difference.
Anyone who handles organic chemicals knows the trouble that poor airflow causes. Allowing 2,4,6-Trimethylphenol vapors to collect in a closed space means trouble for workers’ lungs. Proper local exhaust and regular air exchange reduce risks for everyone—no need for guesswork when real-time air monitors sound an alert the moment something drifts above the safe range. Clean air means healthier, safer work shifts and limits chemical headaches, literally and figuratively.
I’ve seen the results of storing volatile chemicals in the wrong packaging—labels faded, leaks at seams, and corrosion hidden on the inside. The choice of thick HDPE drums or glass jars keeps air out and prevents unwanted reactions. Never store it next to strong oxidizers or acids. Crossing that line even once can spark off fires or generate dangerous fumes that travel far beyond the original container. Secondary containment trays, while sometimes overlooked, catch leaks before they become bigger messes. Sometimes the cost of an extra tray or heavier lid pales compared to a fine or cleanup bill down the line.
Clear, durable labels on every container aren’t there to satisfy some regulatory urge—they really help in emergencies. Crews know exactly what’s inside and how to respond if something spills or catches fire. Relying on memory or makeshift tags opens up room for mistakes. Gloves, goggles, and lab coats aren’t optional either—skin contact can cause nasty irritation. Safety data sheets should be nearby and easy to grab during an accident, guiding fast, targeted first aid.
No storage system stays safe without people who know the hazards. Regular training turns good habits into second nature. Fire extinguishers, showers, and eyewash stations need to work and stay accessible. Spill kits arranged at several points throughout the building cut down response time when seconds matter most. Sprinklers and smoke detectors can be the thin line between a short scare and a disaster that disrupts operations or, worse, causes injuries.
Chemical theft feels like a rare threat, but locking up value chemicals after hours protects not only inventory but also against malicious misuse. Monitoring systems with cameras and logs deter bad actors. Being good neighbors matters, too—giving local firefighters up-to-date storage maps helps them respond smarter and faster in an emergency. Compliance with safety codes reflects more than keeping regulators happy; it builds trust with communities who live just beyond facility walls.
2,4,6-Trimethylphenol, known by those in the lab as mesitol, stands out as a solid that gives off a strong, almost medicinal smell. It looks white or off-white when pure, but impurities can tip it toward yellow. Try handling it without gloves for a few minutes and you'll see why people notice the scent right away. You won’t find it dissolving in water very well, but it blends much better in organic solvents. Anyone working with solvents like ether or alcohol in the lab knows this comes in handy, especially when reactions call for precise mixing instead of cloudy suspensions.
With a melting point sliding in near 44–46 °C and a boiling point cruising around 243 °C, this compound straddles a line between solids and liquids in room temperatures that jump around. Ever had to fish out a bottle from storage on a hot summer afternoon only to see it half-melted? That’s 2,4,6-Trimethylphenol for you, and this behavior shapes how labs store and handle it across seasons. Refrigeration makes sense if you need a solid for weighing, especially in humid places, but above that, you’ll have a liquid that pours easier and reacts faster.
Run a lab test with water, and 2,4,6-Trimethylphenol largely floats around, refusing to budge—less than one gram dissolves per liter. Try the same trick in ethanol or diethyl ether, and it disappears almost completely, making solutions that scientists use for syntheses and industrial purposes. This property shapes safety strategies, too. Cleanup calls for organic solvents, not just soap and tap water, and waste management routes send it through organic recovery channels, not down the standard drain.
Weighing out 2,4,6-Trimethylphenol feels different from similar phenol compounds. There’s a slightly slippery texture, but not quite waxy like naphthalene, and it won’t clump up if humidity stays low. In dry air, it stays crumbly, and static charge doesn’t cause issues with scooping from jars. Those small details matter during high-throughput batch work or in teaching labs, where a single sticky clump means waiting for glassware cleaning or repeating a measurement.
A compound’s melting point and solubility carve out its practical roles and risks. Here, 2,4,6-Trimethylphenol’s moderate boiling point keeps it stable under most heating conditions without sudden vapor bursts, but those volatile aromatic fumes still demand fume hood work. Chemists and students often recall its pungency even after a session ends. This isn’t only a comfort consideration; extended inhalation may cause irritation, a fact underlined by regulatory agencies like the European Chemicals Agency.
Finding practical ways to store and transport compounds that bridge solid and liquid at room temp isn’t academic theorizing. Vacuum-sealing can help slow oxidation and yellowing. Using brown glass bottles cuts down on light-induced changes, especially in bench-top storage. In the classroom, I’ve seen instructors use the physical quirks of 2,4,6-Trimethylphenol to teach lessons about careful handling, storage adaptation, and solvent safety.
Knowledge of the subtle differences—how a slight shift in temperature can turn a powder into a liquid, how an off-white solid can transform after weeks on the shelf—guides both small-scale researchers and large industrial operations. It helps drive home the message that even common organic compounds deserve respect, attention, and an understanding grounded in real laboratory experience.
| Names | |
| Preferred IUPAC name | 2,4,6-Trimethylphenol |
| Other names |
Mesitol 2,4,6-Trimethylphenol 2,4,6-Trimethyl-1-hydroxybenzene 2,4,6-Trimethylhydroxybenzene |
| Pronunciation | /ˌtraɪˌmɛθɪlˈfiːnɒl/ |
| Identifiers | |
| CAS Number | 527-60-6 |
| Beilstein Reference | 1209220 |
| ChEBI | CHEBI:34460 |
| ChEMBL | CHEMBL16322 |
| ChemSpider | 5731 |
| DrugBank | DB03780 |
| ECHA InfoCard | 100.011.077 |
| EC Number | 205-448-1 |
| Gmelin Reference | 126120 |
| KEGG | C02534 |
| MeSH | D014286 |
| PubChem CID | 6977 |
| RTECS number | SL7525000 |
| UNII | 68V6EH8B1N |
| UN number | UN2325 |
| CompTox Dashboard (EPA) | DTXSID1044253 |
| Properties | |
| Chemical formula | C9H12O |
| Molar mass | 150.22 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | phenolic |
| Density | 0.998 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 2.83 |
| Vapor pressure | 0.03 mmHg (25 °C) |
| Acidity (pKa) | 10.89 |
| Basicity (pKb) | 8.38 |
| Magnetic susceptibility (χ) | -62.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.533 |
| Viscosity | 2.23 cP (25°C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 179.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -262.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4607.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364, P501 |
| Flash point | Flash point: 113 °C |
| Autoignition temperature | 515 °C |
| Explosive limits | Explosive limits: 1.1–6.4% |
| Lethal dose or concentration | LD50 (oral, rat): 820 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2960 mg/kg (rat, oral) |
| NIOSH | SD6475000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) of 2,4,6-Trimethylphenol is "2 ppm (10 mg/m³)". |
| Related compounds | |
| Related compounds |
Phenol 2,4,6-Trimethylaniline 2,4,6-Tribromophenol 2,4,6-Trichlorophenol 2,3,6-Trimethylphenol |