Traces of o-ethylphenol turn up far before modern labs had sophisticated chromatography. In the early nineteenth century, curious chemists noticed peculiar odors during coal tar distillation. Over decades, advances in organic synthesis and demand for tailored phenolics in materials science set the stage for isolating and characterizing o-ethylphenol. Once folks realized the unique character of each isomer, especially how subtle shifts on a benzene ring changed odor and reactivity, the groundwork for today’s fine chemical production was secured. As industrial output grew through the twentieth century, so did the need for high-purity aromatic compounds, bringing systematic efforts in scaling up o-ethylphenol while keeping costs and impurities in check.
O-ethylphenol slots into the world of substituted phenols — those aromatic compounds prized for their intermediate status in both fine and bulk chemical industries. Its use traces a line through fragrances, flavoring research, and niche specialty resins. Product forms usually appear as a clear liquid and, to industry insiders, its sharp, sometimes smoky note stands out even among other alkylphenols. Packaging must contend with volatility and sensitivity to light and air. In-shop, operators quickly learn to watch for even minor leaks: A few drops can saturate a room. Buyers regularly seek assurance on purity levels, as trace contaminants can throw entire product runs or risk unsafe exposure thresholds.
A standard bottle of o-ethylphenol might read: CAS 90-00-6, molecular formula C8H10O. Pour it out, and you see a colorless to pale yellow liquid, refractive in strong light. Boiling comes in around 216°C, with a melting point close to the freezing mark for water. Volatility demands good ventilation, and incompatibility with oxidizers or strong acids remains a concern. Its moderate solubility in water means cleanup efforts must stress containment, not dilution. Both its sharp smell and flammability increase workplace caution. I've found storage best in amber glass under inert gas, even short-term, since careless handling promotes oxidation or even peroxidation.
Industry typically asks for o-ethylphenol in concentrations above 99%. High-purity blends mean analysts mark every shipment for acidity, moisture, and possible polymerization. The main regulatory frameworks point to GHS labeling: warnings for acute toxicity, skin irritation, and environmental hazard. In practice, bottle labels reflect a combination of hazard statements, first-aid advice, and details on handling spills or exposure. Accurate barcoding and tracking help prevent mix-ups not just in shipping, but in every warehouse transfer. Lot-to-lot traceability turns out as crucial as any test result. End-users pressing for consistency challenge suppliers to maintain rigorous QA, since small formulation shifts can throw off research results or industrial performance.
Producers often begin with ethylation of phenol, a process that juggles catalyst management and temperature control. Friedel–Crafts alkylation under aluminum chloride or ferric chloride leads the pack. This old-school method delivers a mixture of isomers, so today’s plants rely on fractionation, distillation, and sometimes selective recrystallization for isolation. Stray isomers, over-alkylation, and catalyst residues pose constant headaches on bulk lines. Efficiency and yield tug against process complexity: Lower temperatures limit polymerization but slow the reaction. Purification makes a difference at every step, especially if the material targets analytical labs. Looking at by-products for secondary streams, or integrating recovery systems for spent catalysts, makes both business and environmental sense.
O-ethylphenol doesn’t hide its active para-position; this spot drives electrophilic substitution, letting chemists tack on groups with relative ease. I’ve run nitration and halogenation reactions using o-ethylphenol as the starting block, watching as each tweak changes solubility, volatility, and even toxicity. In polymer labs, cross-linking kicks in for specialty resins. It couples gracefully, especially with diazonium salts in azo dye work. Sometimes it gets hydrogenated, or spun through oxidation cycles chasing quinones or acids. Each manipulation spawns derivatives, used in everything from research standards to bulk intermediates. Capability isn’t so much the question as controlling conditions and cleaning up after, because phenol vapors won’t forgive shortcuts.
Beyond “o-ethylphenol,” catalogues list names like 2-ethylphenol, ortho-ethylphenol, or simply C8H10O. Traders and regulatory lists sometimes call it 1-ethyl-2-hydroxybenzene. These variations matter in regulatory filings, supply chain sheets, and export paperwork. It’s common to find one name on the MSDS sheet, another on a customs form, and yet another on lab stock bottles. Even experienced operators double-check to keep consistency across records. Mislabeling in transit can lead to customs trouble, shipment delays, or, at worst, workplace confusion and serious exposure incidents.
The occupational dangers of o-ethylphenol force any site manager to add extra training. Inhalation brings intense respiratory discomfort; skin contact means quick irritation, even burns. Spill protocols suggest immediate use of chemical goggles, gloves, and fume hoods. Fire risk climbs with vapor pressure, and since local exhaust alone doesn’t always control vapors, operators monitor the workspace with portable detectors. Critical to safety is not just PPE, but robust storage: flameproof cabinets, away from acids and oxidizers, shield from risk. I’ve seen operations tighten controls with regular air monitoring and strict transfer logs. Safety posters only go so far; frequent drills and an open reporting culture impact outcomes more than anything written on a wall.
Once limited to chemical research, o-ethylphenol has climbed into sectors ranging from flavor analysis to advanced polymer manufacturing. The sensory profile, especially its sharp, smoky notes, gets attention in the beverage and flavor industry for off-note detection or modeling complex scents. In polymer chemistry, it provides tailored phenolic backbones for adhesives and coatings, where thermal resistance and specific electrical properties matter. Environmental monitoring programs use it as an indicator of phenolic pollution in industrial runoff. With researchers pushing into more sustainable surfactants and oil recovery materials, o-ethylphenol offers a test bed for new functional groups or performance improvements. Each market segment expects rigorous documentation, not just on purity but on breakdown products and trace residues.
Discovery in o-ethylphenol hasn’t slowed: academic groups publish on catalytic improvements each year, while major chemical firms fund tweaks in separation and scale-up. Environmental chemistry asks about biodegradation: “How long does it stick around? Is it building up?” Analytical labs find novel uses profiling trace levels in foods and beverages, especially since consumer palates react to parts-per-billion signals. Tech hubs push for computer modeling of reactivity and degradation, driving innovation in predictive tools for process chemistry. Unlocking cheaper, cleaner routes to o-ethylphenol could lower costs across entire industry verticals, benefiting everyone from large manufacturers to boutique research teams. Industry-academic partnerships have driven forward leapfrog advances, especially in catalyst recovery and waste minimization.
Serious questions still swirl about o-ethylphenol’s impact at low doses, especially for workers with long-term exposure. Acute symptoms—dizziness, skin burns, respiratory distress—turn up fast if controls lapse. Chronic toxicity studies link exposure to liver and kidney changes in animal models, though translating findings to human risk takes more data. Regulatory agencies set thresholds for workplace air, and labs keep tracking metabolites after trace ingestion. In my own experience, even brief lapses in glove protocol can leave lasting reminders for days. Toxicologists continue investigating breakdown products, with growing emphasis on low-dose, long-exposure syndromes. Public health advocates push for tighter monitoring, calling for transparent sharing of research and prompt revisions to exposure limits.
Looking ahead, shifting regulations may force industry to rethink both synthesis and downstream use of o-ethylphenol, especially as safer substitutes edge into the market. Greener routes, lower-waste processes, and biodegradable derivatives appeal not only to regulators but to companies anxious about corporate responsibility claims. Research groups now assess potential in new heat-resistant polymers, advanced composites, and hybrid materials for energy storage. Universities run small pilot projects exploring bio-based feedstocks and more benign routes to key phenolics. On the application side, the balance hangs between traditional uses—where consistency and performance rule—and emerging sectors eager to minimize environmental and worker risks without sacrificing utility. Conversations in boardrooms and labs turn more and more on traceability, transparency, and lifecycle analysis, charting the long-term pathway for o-ethylphenol-based innovations.
O-Ethylphenol shows up in more places than people might guess. Chemists know it as a colorless liquid with a distinct, sharp odor, but its impact runs deeper than just its aroma. O-Ethylphenol comes from chemical reactions involving phenol and ethyl compounds. Once made, this compound finds its way into several industries. My time in fragrance labs taught me that O-Ethylphenol acts as a reliable aroma component, giving fragrance formulas a smoky, spicy punch. That edge isn’t just for perfume; it touches everyday products from cleaning sprays to scented candles. O-Ethylphenol brings authenticity and body to scents that aim for a lived-in, almost woody feeling.
People often drink O-Ethylphenol, though rarely on purpose. This compound shows up naturally in some drinks and food. Most famously, red wines depend on subtle O-Ethylphenol traces to add depth and complexity. Sometimes too much leads to unpleasant flavors, described by wine experts as “barnyard” notes. Controlled, though, it helps create memorable bottles. Brewers face the same balancing act. Belgian beer relies on the compound for signature earthy overtones. Craft brewers often talk about chasing the right amount—enough to add character, not enough to overpower. From my experience working alongside brewers, quality hinges on close monitoring of levels using gas chromatography. This technology helps keep the brew palatable, respecting both tradition and modern standards for taste and safety.
This compound has more influence off the dinner table. In chemical manufacturing, O-Ethylphenol sits at an important step for building other useful chemicals. That side of production matters even if it happens out of sight for most people. O-Ethylphenol helps create antioxidants and stabilizers, substances that keep plastics, rubbers, and even food fresh longer. Plastic manufacturers use it as an intermediate to give finished goods strength and chemical stability. Simple as it sounds, small choices like which intermediate to use can change whether a product lasts or breaks down early. In my years supporting industrial research, I watched teams focus closely on ingredient sourcing and handling. Clean production lines matter as much as strong regulations, since trace contamination from O-Ethylphenol can impact product quality.
Working with chemicals comes with risk, and O-Ethylphenol is no exception. Its strong smell signals caution—prolonged exposure might irritate eyes and lungs. Industries must approach its use with strict safety gear, smart ventilation, and constant monitoring. Regulatory agencies like the EPA and workplace safety boards offer guidelines meant to protect handlers and communities. I’ve sat in on safety workshops where the focus was always on practical steps: clear labeling, routine checks, and quick access to material data sheets. Proper disposal also matters. Specialty facilities process O-Ethylphenol waste, keeping pollution risks in check. Companies that invest in staff training and collaborative oversight help minimize danger while continuing to benefit from O-Ethylphenol’s unique properties.
O-Ethylphenol offers benefits, from improving scents to supporting durable plastics. Still, paying attention to environment and health risks makes sure these gains aren’t lost to oversight or neglect. Investing in high-quality measuring tools and focusing on cleaner processes stands out as the practical path. Each step, from lab bench to production floor, impacts the final result. Keeping a close watch, sharing new research, and following strict safety routines help ensure O-Ethylphenol continues to serve in ways that matter, both in industry and daily life.
Working with chemicals like O-Ethylphenol isn’t just about following rules—it’s about protecting your coworkers and yourself from harm that nobody wants to experience. O-Ethylphenol, a compound used in synthesis and research, carries hazards that can turn a routine day upside down without the right precautions. Even at low levels, it releases vapors that irritate eyes, nose, throat, and skin, and long-term exposure may affect health in ways that are hard to reverse.
My time in chemical research taught me that gloves alone won’t do the trick. O-Ethylphenol penetrates some glove materials surprisingly fast. We swapped out latex for nitrile gloves, which stand up better against spills. Goggles or wrap-around safety glasses only came off when everyone agreed it was safe again; a tiny splash in the eye leaves a sting you’ll remember for months.
Every lab worth its salt keeps a set of dedicated coats—not just any fabric, but dense cotton, so if something splashes, skin exposure stays minimal. Short sleeves never make sense here. Cover as much as possible, and change out of contaminated clothes before heading home or, worse, into a public area.
Ventilation isn’t a luxury. It’s a necessity. Those who have tried to handle O-Ethylphenol outside of a fume hood know how quickly fumes fill a room. Fume hoods, if properly maintained, remove airborne hazards at the source. Always check the airflow with a strip of tissue or similar before starting anything sensitive.
Keeping O-Ethylphenol locked down in airtight containers protects not just against accidental whiffs. Volatile chemicals drift, and before you notice, the whole workspace smells, even though the main bottle looks sealed. Store it in flammables cabinets away from sunlight or heat, as this reduces both spill risk and the danger of an unexpected fire.
Spills freak people out, but a plan transforms panic into action. We kept spill kits with absorbent pads, neutralizing agents, and a clear step-by-step chart right by the entrance. Practice goes a long way here. Actual incidents show that people hesitate mostly from uncertainty. Drills help everyone lock in the steps: contain, absorb, bag, and report.
Bottling up waste in labeled, sealed cans keeps janitors and environmental services from nasty surprises. Never pour it down the sink—municipal treatment plants aren’t built to handle phenolic contaminants. Licensed chemical disposal services keep everyone out of trouble with regulators and, more importantly, the water supply safe for the whole town.
No manual replaces hands-on training. Walking new staff through safe handling and emergency response makes a bigger difference than any number of memos. Ask questions, revisit steps, and keep communication open about anything that didn’t go as planned. A culture where people actually speak up helps catch problems early—usually before curious paperwork or hospital visits ever enter the picture.
The Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH) point to these same principles. Published studies have shown that workplaces investing in both training and equipment see lower rates of chemical exposure incidents. Respect for the chemical, blended with solid habits, builds a team that doesn’t just avoid mistakes but sets a higher standard for every lab session.
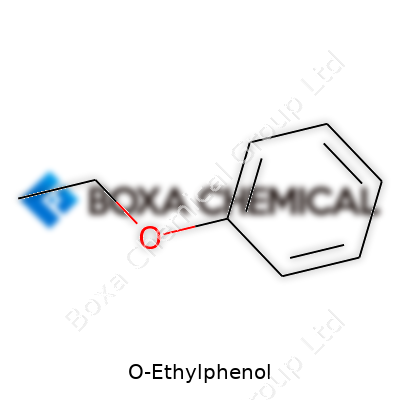
O-Ethylphenol often comes up for anyone exploring organic chemistry, perfumery, or the world of food and beverage science. Its chemical structure grabs my attention every time because small tweaks change what molecules can do, how we perceive them, and how safe they are for our communities. Everyday chemistry lessons from old labs taught me molecules get their attitude from structure. O-Ethylphenol’s formula is C8H10O. That looks plain at first, but plenty sits behind those letters and numbers—especially compared to its close chemical cousins.
In O-Ethylphenol, an ethyl group attaches to the oxygen atom on a benzene ring with a hydroxyl group in the ortho position. For people outside labs, think of this as slight shuffling of features on a familiar face. Substitute just a couple of atoms, and you get different odors, solubilities, toxicities, and uses. This can mean the difference between safe aroma compounds in whisky or dangerous contaminants in water systems. The formula, C8H10O, still represents a compound that can swing between friendly and problematic, depending on where those pieces sit. That matters for everyone in the supply chain, from growers and producers to folks doing regulatory checks.
O-Ethylphenol turns up naturally in some fermented foods and drinks—especially in aged spirits, where it can bring out flavors people call “barnyard” or “leather.” Some of my friends describe certain whiskies by these distinct tasting notes. Brewers and distillers use this knowledge to fine-tune flavor, making the chemical’s identification and control essential to both protecting brands and meeting safety codes. On the other hand, trace amounts in water supplies might raise alarms for public health officials, so accurate chemical formulae help them enforce standards and keep communities safer.
Problems come up if standards slip or if detection methods don’t catch everything. Regulators and companies spend many hours developing rigorous testing, especially for products meant to be consumed or released into the environment. From research, I’ve seen inconsistencies in monitoring standards across different regions. That puts some populations at greater risk, especially where funding or training in analytical chemistry falls short. Poor chemical literacy among the general public also means some communities can be blindsided by a compound’s risks or miss out on its benefits.
Better chemical education changes the game. If more people learn why formulas matter—like C8H10O for O-Ethylphenol—they become informed advocates for their own health and safe consumer choices. At the same time, funding goes a long way in keeping lab instruments up-to-date and researchers trained on the most effective analysis techniques (such as gas chromatography-mass spectrometry). Collaboration between local agencies, universities, and producers can help keep harmful levels out of products and the environment. Updated databases, open to public review, foster trust and transparency.
Knowing the formula C8H10O is more than memorizing a line in a textbook. It serves as a tool for quality control, health protection, and even creative expression in food science. With accurate science, open communication, and hands-on chemistry training, both industry and public interests can stay balanced in the treatment and use of O-Ethylphenol.
O-Ethylphenol may sound like another ordinary lab chemical, but anyone who’s ever handled organic solvents knows cutting corners on storage can end badly. O-Ethylphenol carries a strong odor, evaporates quickly, and burns if it catches an ignition source. Spills at room temperature fill the air with fumes that irritate the nose and throat. Some storage mistakes turn a routine day into a safety scramble.
I spent three years working in quality control at a fragrance lab. O-Ethylphenol arrived in heavy drums from overseas suppliers, and new technicians sometimes treated it like harmless flavoring. A single misplaced container could make the whole warehouse smell sweet and sharp for a week. Worse, barrels with loose lids let fumes build up. One time, a drum got stashed closer to a boiler in winter; the vapor smell grew in the back hallway, and the fire marshal almost fined us for improper storage. That lesson cost real money and goodwill.
Keeping O-Ethylphenol under control means thinking ahead. The compound stays stable in sealed containers, out of direct sunlight. Bright light, especially heat from sunlight, does more than fade labels—the liquid evaporates faster and might pressure the container. Storing it in a flammable storage cabinet reduces risk. National Fire Protection Association (NFPA) codes call for proper segregation of flammable liquids, and insurance policies demand the same. OSHA’s rules require that flammable chemicals like this one stay in cabinets or designated rooms, never on open shelves or in corridors.
Some folks skip secondary containment pans, treating them as overkill. In reality, catch pans stop drips and spills from spreading underfoot or into drains. After seeing a colleague trip over a slick floor, I learned to appreciate splash guards and containment.
During drum transfer, workers need gloves and goggles. Skin exposure leads to redness and irritation; splashes in the eyes sting badly. Lab coats protect clothes, but aprons give better results during cleaning or decanting. It’s surprising how fast these liquids soak through cotton, so I always grab gloves developed for solvent resistance—like nitrile or neoprene—before handling any open container.
Ventilation matters just as much as barriers. Poorly ventilated rooms soon carry a thick, unpleasant scent, reminding everyone of the volatile nature of O-Ethylphenol. Opening a few windows doesn’t make up for an actual chemical fume hood or exhaust system. I remember one facility upgrade that brought air quality up to code, which helped with both comfort and worker health.
Clear labeling stops confusion between lookalike chemicals. Nobody wants a mix-up between O-Ethylphenol and another aromatic in adjacent bottles. Bright warning stickers and up-to-date safety sheets work better than hoping everyone remembers by smell alone. If everyone—from seasoned chemists to new hires—knows the protocol, daily lab life runs smoother. Regular training, not just a single annual meeting, keeps safety habits from slipping. Keeping equipment in top shape and checking storage spots for leaks or damage shouldn’t be left for tomorrow. Taking care today means avoiding costly emergencies.
O-Ethylphenol offers unique value in industry, but the hazards call for practical measures. With the right steps, workplaces stay productive, safe, and prepared for surprises.
O-Ethylphenol shows up in industrial settings, labs, and environments where chemicals get handled often. It belongs to the class of alkylphenols—a group recognized for their role in manufacturing, plastics, and sometimes pesticides. Its strong, distinct odor often works as a warning sign, though many workers encounter it long before any discomfort starts.
The most striking health risks link back to inhalation or skin contact. Short exposure leads to eye and throat irritation, headaches, and nausea. Longer-term or higher-concentration exposure might cause symptoms that point to deeper trouble, like dizziness, coordination problems, or confusion. The skin takes the brunt of spills. Redness, dryness, and even burns show up when safeguards slip.
Lab studies with animals point to impacts on the liver and kidneys after heavy doses, and O-Ethylphenol can mess with hormone function the way some industrial chemicals do. Though researchers haven’t nailed down all the long-term effects in humans, the worry builds when workplaces repeat exposures without enough control.
Years spent in industrial safety show me that workers rarely get exposed to just one chemical. Usually, O-Ethylphenol comes surrounded by cousins—other volatile organics, plastic additives, or solvents. Once, a routine inspection at a resin plant found a valve leaking vapors near a spot where staff took breaks. No alarms, just headaches and eye pain no one could trace until someone asked the right questions. Everyone on that shift learned fast how chemical exposure sneaks up where attention drifts.
Workers face health risks not just from accidental spills but through small leaks, vapors, or even contaminated gloves. This chemical doesn’t just irritate. It brings more ticking-time-bomb worries, like potential links to hormone disruption. Scientific papers keep chipping away at these links, building stronger cases for caution even at levels below what old guidelines considered “safe.”
Communities near manufacturing plants feel a ripple effect. Air monitoring around these sites sometimes picks up low levels of O-Ethylphenol, raising questions about safe boundaries for families living nearby, not just those clocking in on site. The U.S. National Institute for Occupational Safety and Health (NIOSH) lists this group of chemicals as hazardous and calls for strict engineering controls and personal protection.
The standard fixes—ventilation, gloves, face shields—make sense, but they only work when everyone pays attention and keeps equipment in good shape. Many smaller companies lag on upgrades, pinched by tight margins or poor training. Investing in modern air monitoring and frequent risk training brings lasting returns—fewer hospital trips, lower insurance costs, and much more confidence among employees.
Strong labeling, quick spill cleanup, and regular health checks matter too. It boils down to a workplace culture where no one treats safety like an afterthought. For communities, tighter monitoring near manufacturing sites and clear communication about chemical risks help prevent slow-building health issues. Local advocacy, with help from environmental health agencies, pushes for transparency and swift fixes long before problems pile up.
O-Ethylphenol isn’t just a technical hurdle. It’s a test of how industry values the health of its workers and neighbors. Solutions exist—real ones, not just words on safety posters—only when everyone treats chemical risks as real and urgent.
| Names | |
| Preferred IUPAC name | 1-Ethoxy-4-methylbenzene |
| Other names |
2-Ethylphenol o-Ethylphenol |
| Pronunciation | /ˌoʊˌiːˈθɪlˌfiːˈnɒl/ |
| Identifiers | |
| CAS Number | 90-00-6 |
| Beilstein Reference | 806254 |
| ChEBI | CHEBI:27741 |
| ChEMBL | CHEMBL156055 |
| ChemSpider | 16082 |
| DrugBank | DB02170 |
| ECHA InfoCard | 100.008.926 |
| EC Number | 202-806-2 |
| Gmelin Reference | 613402 |
| KEGG | C02341 |
| MeSH | D010025 |
| PubChem CID | 8232 |
| RTECS number | SJ8575000 |
| UNII | F9IVM8Y1GM |
| UN number | UN2529 |
| CompTox Dashboard (EPA) | DTXSID5021083 |
| Properties | |
| Chemical formula | C8H10O |
| Molar mass | 122.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Phenolic |
| Density | 1.02 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 1.98 |
| Vapor pressure | 0.25 mmHg (25°C) |
| Acidity (pKa) | 10.29 |
| Basicity (pKb) | 9.95 |
| Magnetic susceptibility (χ) | -70.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.529 |
| Viscosity | 5.77 mPa·s (20 °C) |
| Dipole moment | 1.55 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -121.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3787.4 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | H302 + H312 + H332, H319, P261, P280, P305 + P351 + P338, P337 + P313 |
| Flash point | 107 °C |
| Autoignition temperature | 715 °F (379 °C) |
| Explosive limits | 1.2–1.9% |
| Lethal dose or concentration | LD50 oral rat 707 mg/kg |
| LD50 (median dose) | LD50 (median dose): 620 mg/kg (rat, oral) |
| NIOSH | UR0180000 |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 250 ppm |
| Related compounds | |
| Related compounds |
Phenol o-Cresol Guaiacol |