People started working with hydroquinone compounds in the 1800s while exploring natural and synthetic antioxidants. Methylhydroquinone entered the stage as researchers looked to tweak the original hydroquinone molecule for better stability and performance. The early days saw its use tied mostly to photography and dye manufacturing. Over the decades, chemists saw that adding a methyl group to hydroquinone altered its reactivity in ways that helped control oxidation processes. This shift invited more focus on its commercial production, paving the way for cleaner syntheses, improved yields, and stricter quality standards as health and environmental concerns grew.
Methylhydroquinone, often abbreviated as MHQ, grew out of the need for antioxidants and polymerization inhibitors. Most people in chemical industries recognize its solid, crystalline form, which resembles white or off-white flakes. The product usually comes tightly packaged to avoid moisture and light, both of which provoke rapid degradation. Manufacturers often market it under names like 2-Methylhydroquinone or 2-Hydroxy-5-methylphenol. International suppliers and local vendors alike have moved to digital platforms, so sourcing has become more transparent. Labels lean heavily on hazard communication and batch traceability; the information is straightforward and meant to simplify safe handling.
Fresh batches of methylhydroquinone spark curiosity because of the way it melts at roughly 109–111°C and dissolves in alcohol and ether but barely touches water. Its chemical structure packs a punch: a benzene ring with two close hydroxyl groups and a methyl tail sitting in the meta position. This gives the molecule more oil solubility and a bump in antioxidant strength compared to plain hydroquinone. Chemists appreciate how it forms colored quinones with oxidants and holds up under mild handling, although it will brown with enough air.
Chemical suppliers focus on high assay percentages, often listing purity above 98%. Labels spell out CAS number 95-71-6, precise melting range, and potential byproducts—traces of methylcatechol or dimethylhydroquinone. Lots often come with certificates of analysis and MSDS documentation, reflecting rising demands from end users and regulators. The shelf-life depends on package integrity and temperature, with dark, dry storage preserving its practical lifespan. Chemists in the field check for color consistency, melting range, and low water content before clearing product for use.
Industrial-scale production usually follows the methylation of hydroquinone using dimethyl sulfate or methyl halides, catalyzed by strong bases in controlled conditions. The reaction mixture is neutralized, extracted, and crystallized. The process doesn’t go unchecked—scrubbers and wastewater treatment are key in modern plants to address environmental liabilities. Almost every experienced chemical operator learns to monitor pH, reaction time, and solvent recovery at each stage to reduce waste and maximize yields. Smaller labs sometimes draw on alternative methods, like direct hydroxylation of methylphenol, but scaling up always centers on worker safety and environmental compliance.
Methylhydroquinone’s reactivity makes it useful in oxidation and polymerization inhibition. It stands up to dilute acids and alkali, but oxidizing agents flip it quickly into quinones, forming strong brown or red stains familiar to anyone experimenting with aromatic oxidations. It couples with diazonium salts, aiding in dye synthesis. Experienced chemists often test methylation on the side to adjust physical properties, while those in polymer research attach bulky groups to tailor the molecule for new uses in plastics and resins. Hydrogenation turns it back to the corresponding methylcyclohexanediol, broadening its footprint in specialty chemicals.
Industry professionals run into methylhydroquinone under a variety of names: 2-Methylhydroquinone, 2-Hydroxy-5-methylphenol, and 5-Methylbenzene-1,4-diol among the common labels. Those working in European markets sometimes find “MeHQ” or “MHQ” on bulk shipments. Academic researchers reference the parent compound formula C7H8O2 in journals. Distributors stamp trade brand names and supplier codes to track lots through international supply chains, but everyone treats the core product with the same caution.
Workers handling methylhydroquinone wear gloves, splash goggles, and use chemical fume hoods, knowing that skin and respiratory exposure can provoke irritation. Spills are tackled right away, not left to dissipate, since airborne dust and residue lead to long-term workplace contamination. Material safety datasheets recommend using nitrile gloves and proper respiratory filters. Strict disposal rules apply; waste streams with even small MGQ residues go for incineration or specialized chemical neutralization. Regular health monitoring and air sampling reinforce a safety culture in plants that handle high volumes. Training new employees involves hands-on demos, as overconfidence can lead to careless exposure or accidents.
Methylhydroquinone finds its largest uses in resins, rubber manufacturing, and as an inhibitor in monomer storage. Polymer chemists rely on it to stall premature crosslinking reactions in acrylic monomers, ensuring storage tanks stay stable until the right trigger is applied. Researchers in photographic chemistry still use it to develop fine-grained black-and-white prints. A few medicinal chemistry groups explore its antioxidant functions for topical agents in dermatology, though drug regulators watch carefully for side effect profiles. Art conservators and dye makers dip into methylhydroquinone to stabilize colors and prevent pigment degradation over time. It also turns up in specialty coatings and adhesives, where controlled polymerization spells the difference between a shelf-stable product and an unusable blob.
Recent work in academic labs zeroes in on methylhydroquinone’s redox cycling, which models cellular oxidative stress. Investigators use it as a tool to study how cells counteract oxidative damage, feeding into broader drug development projects. Polymer scientists keep looking for new derivatives that perform tougher antioxidant roles or resist UV-induced breakdown. With environmental alarms ringing louder, R&D teams have begun developing greener syntheses, aiming to phase out toxic methylating agents and recycle effluent. Regulatory agencies pressure manufacturers to run repeated chronic exposure studies, checking environmental persistence and breakdown. International partnerships now shape R&D pipelines, with data sharing smoothing the way for safer, more sustainable methylhydroquinone processes.
Animal studies and repeated in vitro screens flag methylhydroquinone as moderately toxic at higher doses. Workers exposed to dust or vapors sometimes report irritation—nose, lungs, and skin take the brunt. Long-term exposure can tip the liver and kidneys into dysfunction, especially without personal protective equipment. Chronic studies in rodents show some cancer risk under high-dose, prolonged use, which keeps regulatory thresholds strict. Environmental researchers find MHQ and its byproducts work their way into wastewater, where breakdown is not guaranteed. Ecological impact studies urge caution, prompting wastewater treatment plants to look for better capture and destruction methods. Data collection on human exposure remains patchy, but most chemical safety boards assume a precautionary stance, favoring conservative exposure limits.
Companies face a real squeeze balancing utility and responsibility with compounds like methylhydroquinone. Technology drives demand for antioxidants with longer shelf lives and better performance under heat or UV exposure, putting this molecule in the crosshairs for digital printing, 3D-printed polymers, and functional coatings. Chemists look for machine learning models to predict better derivatives or greener syntheses. Waste minimization and regulatory transparency attract venture capital and public funding for process improvement. European and Asian markets, historically slow to embrace new hazard guidelines, have begun syncing standards for storage, transport, and labeling, improving global accountability. Training programs now fold in green chemistry modules for new hires, fueling a generation that expects safer, cleaner production. The chemical’s story is far from over—driven by demand, risk management, and the steady march toward sustainable industrial chemistry.
Methylhydroquinone stands out as a chemical with multiple faces. Often called 2-methylhydroquinone, it comes from the hydroquinone family, known for their role as antioxidants. Places like laboratories and factories see a steady demand for it because of the way it reacts to oxygen and light. That quality puts it on the list of chemicals shaping modern industry and products. People involved in science or research run into it fairly early in their careers, usually through textbooks or lab demonstrations.
Factories put methylhydroquinone to work as a stabilizer and antioxidant, a trait it shares with other hydroquinones. In rubber production, this compound protects raw materials from spoiling due to oxidation. Rubber needs to stay flexible and durable. Spoiled rubber costs businesses money and damages reputations. Watching factory workers handle methylhydroquinone—carefully measuring tiny amounts, wearing gloves and goggles—drives home just how much has to go right for tires or balls to reach store shelves in good condition.
Old-school black-and-white photography used to depend on hydroquinone derivatives. Their ability to make images appear on film made developing rooms seem almost magical to anyone who tried the process. Some electronic applications—like making specialized semiconductors—make use of methylhydroquinone as part of the cleaning and etching process. Clean circuits and sharp photographs may seem unrelated, but chemistry means both depend on how a single compound reacts with other elements under set conditions.
Handling methylhydroquinone in the workplace means more than following directions. People who work in chemical plants do not want surprises—bad ventilation, careless spills, or rushed procedures. Health experts recognize that the compound can irritate skin, eyes, and the respiratory system. Long-term, repeated exposure brings up even more questions about safety. Households shouldn’t keep methylhydroquinone on hand; its use asks for professional oversight, strict labeling, and responsible disposal.
Environmental groups keep a close eye on where and how chemical waste from industries gets dumped. Methylhydroquinone should never end up in waterways. Wildlife reacts to even small traces. It is part of a bigger story about why proper waste management matters for everyone, not just those who work with chemicals directly.
Newer studies look for substitutes that work just as well but with less risk. Science keeps evolving, inching toward safer product lines and gentler processes. Regulators and industry leaders need to listen to scientific advice, invest in worker safety training, and keep an open line with communities near manufacturing sites. In my experience working with environmental advocates, dialogue between industry and neighborhoods brings better outcomes than secrecy or neglect.
Methylhydroquinone’s importance stretches beyond its immediate use. It marks a crossroads between invention, workplace safety, profit, and community health. As more alternatives become available, and as new generations step into science and industry jobs, the story will keep changing. Everyday choices—from the materials in sporting goods to the technologies powering new gadgets—connect back to decisions made about chemicals like methylhydroquinone.
Looking at the lengthy ingredients on skincare bottles, I usually reach for my phone to check what they really mean. Methylhydroquinone catches the eye for good reason — it shows up in some lightening creams, and it sounds similar to hydroquinone, the whitening compound banned in several countries. People use these products chasing clearer and brighter skin, but not every shortcut deserves your trust.
Methylhydroquinone comes from the same chemical family as hydroquinone, but adding that “methyl” changes how it behaves. Hydroquinone acts as a strong skin lightener, blocking melanin production. It’s so effective that some governments don’t allow over-the-counter sales, linking it to potential long-term skin damage, increased risk of ochronosis (a blue-black discoloration), and even possible cancer risk with prolonged use. Methylhydroquinone shows up less often in common cosmetic products, but the structural similarities raise red flags for dermatologists and scientists.
As of now, peer-reviewed research on methylhydroquinone lags far behind its cousin hydroquinone. Toxicology studies point out that adding a methyl group doesn’t always make a hazardous chemical safer. A handful of experiments suggest methylhydroquinone may still generate free radicals under sun exposure — not great news for skin health. Chronic exposure to related compounds sometimes sparks allergies or worsens sun damage.
The American Contact Dermatitis Society and leading dermatologists always refer back to real cases of irritant and allergic reactions. Hydroquinone, in the right percentage and with careful use, helps certain skin conditions under medical supervision, but authorities never green-light its unchecked use. There’s even less reason to trust methylhydroquinone, given the lack of oversight and large-scale safety studies.
No healthy skin fix ends at bleaching agents. Small cosmetic brands might add methylhydroquinone without testing its effects in people with different skin tones or sensitivities. Unregulated products ship across borders. In some places, people fall into the trap of trading safety for fast results, only to develop worse problems down the line, including scarring or permanent pigment changes.
Choosing skincare for healthy results means looking for ingredients that studies support, not just chasing the newest thing on a label. Vitamin C, niacinamide, retinoids, and regular sunscreen all have research behind them, with dermatologists guiding safe use. People deserve better options than risking their health chasing quick fixes, especially when the benefit isn’t clear-cut and the risks remain poorly understood.
Regulatory agencies and health professionals need to push for transparency. Requiring cosmetic companies to share more about what’s in their products and how those chemicals behave would close the gap between curiosity and knowledge. Until long-term studies document real-world safety, it makes more sense to steer clear of mysterious ingredients like methylhydroquinone and trust tried-and-true advice from those who spend their lives studying skin health.
Methylhydroquinone often pops up in the conversation about skin lightening products. Plenty of people reach for creams that promise a lighter complexion, not always aware of the long-term health cost. I’ve seen folks use these creams without reading labels or having doubts. Methylhydroquinone doesn’t just fade dark spots — it can stir up trouble much deeper than the skin.
I remember overhearing a pharmacist explain to a woman about why some creams aren’t just about vanity. He spoke about methylhydroquinone and asked if she had any allergies. Turns out, she hadn’t even known what was in her cream. Common side effects include skin irritation, redness, and burning sensations. At its extreme, skin becomes thin and fragile. People find themselves with a patchy, mottled look called ochronosis—the very opposite of what they set out to get.
Medically, there’s more trouble hiding beneath the surface. Chronic exposure, especially without doctor supervision, can weaken the skin’s natural barrier. This drives up risks for infections because the protective layer just isn’t there anymore. The structure of methylhydroquinone shares chemical relatives with hydroquinone, which has been banned in several countries over safety worries.
Allergic reactions aren’t rare. Rash, swelling, itching, or hives may show up, sometimes just from a few applications. People with sensitive skin or a history of allergies face even higher odds of a reaction. It’s a gamble many take without understanding the odds.
Here’s a point not enough people consider—the possibility of absorption into the body. Most think a topical cream remains on the skin, but certain chemicals pass through and enter the bloodstream, especially with repeated use. Toxicity, although less common, can crop up when people use these products over large skin areas or for months at a stretch. Lab studies have raised questions about links to organ damage and even cancer with long-term exposure, adding to reasons for stricter regulation in Europe and parts of Asia.
Long-term use also affects mental health. The pressure to change skin color, combined with visible side effects like ochronosis or permanent scarring, chips away at self-confidence. In my own community, several people struggled not just with the physical consequences, but also feelings of regret and anxiety over irreversible changes.
Solutions start with awareness. Ingredients like methylhydroquinone deserve a strong warning label. Dermatologists urge people to skip self-prescribing and to get professional advice. Testing a small patch of skin before full application helps spot reactions early. It’s smart to check government advisories about these chemicals and seek alternatives. Some plant-based brighteners offer safer results for milder concerns.
Beauty standards put a heavy load on people, tempting them toward risky shortcuts. Education, tougher regulation, and honest conversations with healthcare providers beat back misinformation. There’s no single fix, but informed choices stack up to better health, inside and out. Recognizing these risks can push the conversation away from chasing instant results toward safeguarding long-term well-being.
Anyone who spends time working in chemistry labs or manufacturing facilities knows how certain compounds can go from useful to hazardous in the blink of an eye. Methylhydroquinone is one of those materials. Its applications in hydrogen peroxide production, photography, and polymers sound routine until something’s off with how it’s kept. Once, I watched a drum that got a little too warm—from being near a sunny window—start to bulge. That was enough of a wake-up call for everyone present.
Methylhydroquinone isn’t just another chemical that sits on a shelf quietly. It oxidizes easily if it meets air or temperatures higher than recommended. This transformation usually means less effectiveness, but in the worst-case scenario, it can mean catching fire. Chemical Safety Facts and PubChem outline its tendency to self-heat and even combust, especially if it's impure. Those facts line up with what regulators say: store it cool, dry, and tightly sealed.
After seeing what a simple mistake can create, I never underestimate storage instructions. For methylhydroquinone, I keep things simple but strict:
Facility managers or anyone handling chemicals often take guidelines for granted. Yet, these rules have real consequences: the Occupational Safety and Health Administration (OSHA) documents regular injuries from improper chemical storage every year. Even one slip—like putting methylhydroquinone near a heat vent—brings real harm to people and property.
Manufacturers and labs often rely on training sessions, but reminders only go so far. Automated temperature loggers, regular walk-throughs, and checklists keep standards from slipping. In my experience, peer-to-peer walkthroughs uncover more blind spots than top-down inspections. Here’s where digital systems play a role. QR codes on storage areas can link directly to hazard sheets and up-to-date SOPs for anyone on the floor, making information handy and action more likely.
Investing in storage cabinets designed for hazardous chemicals can seem costly, but over time, it prevents spoilage and injury. I’ve seen organizations lose ten times the price of safe storage on wasted product and fines after a breach. The up-front work pays off.
Nobody benefits from confusion during a workplace emergency. That’s why talking over the procedures, reviewing hazards during team meetings, and updating signage always finds its way into my routine. The Centers for Disease Control and Prevention recommends always keeping staff informed on what’s in stock and how to handle every container. Information empowers action and keeps workplaces safe.
The bottom line comes down to respect: for people, the tools they use, and the places they work. Safe storage for methylhydroquinone isn’t just a line in a safety manual. It’s good science and common sense that help everyone head home after a shift in one piece.
People have been chasing lighter and clearer skin for ages. Methylhydroquinone shows up in a lot of over-the-counter products targeted at fading dark spots, evening out skin tone, or tackling stubborn marks from acne. Like its better-known cousin, hydroquinone, this compound interrupts the process that gives skin its color. The difference? Methylhydroquinone gets less media attention, but shoppers see its name listed on labels, often alongside bold claims and big promises.
You walk into a pharmacy in the U.S., searching for skin lighteners, and staff direct you to a locked cabinet or cosmetic aisle. Most people, myself included, remember spotting creams with hydroquinone right on open shelves pre-2020. That changed when the FDA reclassified hydroquinone as a prescription drug. The concern? Burning, redness, permanent discoloration, and the possibility of worse. Health agencies around the world responded to consumer complaints with tighter restrictions.
Methylhydroquinone sits in a gray area. I’ve talked with pharmacists who keep tracing the rules, since regulations shift from one country to another. In the U.S., any ingredient that acts in the same manner as prescription-only hydroquinone is likely viewed the same way by regulators. So, even though you might stumble across “brightening agents” on beauty websites, the law treats potent actives seriously. Pharmacies err on the side of caution, often requiring a prescription for any product with a high level of methylhydroquinone.
Here’s reality: not all substances are created equal when it comes to safety. Medical experts—from dermatologists to toxicologists—agree misuse brings real risks. Using methylhydroquinone without a doctor’s guidance exposes people to possible chemical burns, allergic reactions, or even long-term changes in skin color that don’t fade away. Unregulated creams from random websites or abroad often dodge proper ingredient lists or strength disclosures, making matters even worse. Stories of hospital visits caused by mystery creams show up every year.
This isn’t just a skin-deep issue. Unchecked use of agents like methylhydroquinone connects to bigger public health concerns. Some batches can be contaminated with dangerous substances. The World Health Organization and other watchdogs urge stricter monitoring, especially in regions where skin lightening still flies under the radar.
Sometimes, people searching for solutions don’t realize the risks. Easy online access tempts buyers, but I’ve seen the regret up close among friends who thought a simple cream would erase dark patches and found themselves battling side effects for months. Instead of making risky guesses at home, connecting with a dermatologist opens doors to safer, science-backed options.
Transparency matters too. Beauty brands with nothing to hide should share concentrations, potential dangers, and the science behind their claims. Retailers and e-commerce platforms can step up screening and block unproven imports from slipping through. Lawmakers need to keep reviewing these products as new data comes out, since the skin care market evolves much faster than rules sometimes keep up.
Consumers deserve tools for fact-checking. More straightforward public health campaigns, led by trusted experts, could clear up the confusion. With honest guidance and expert care, shoppers have a better shot at healthy skin and fewer regrets.
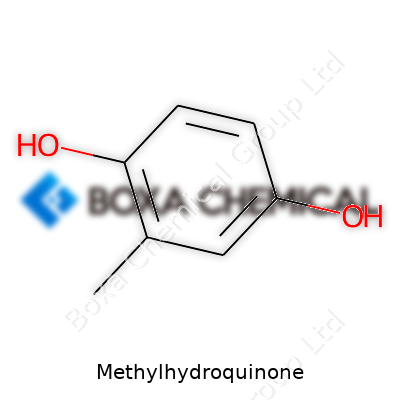
| Names | |
| Preferred IUPAC name | 4-Methylbenzene-1,2-diol |
| Pronunciation | /ˌmɛθɪl.haɪdrəʊ.kwɪˈnəʊn/ |
| Identifiers | |
| CAS Number | *150-76-5* |
| Beilstein Reference | 1209240 |
| ChEBI | CHEBI:18002 |
| ChEMBL | CHEMBL15737 |
| ChemSpider | 55776 |
| DrugBank | DB13356 |
| ECHA InfoCard | 100.017.875 |
| EC Number | 205-776-6 |
| Gmelin Reference | 8486 |
| KEGG | C07185 |
| MeSH | D008768 |
| PubChem CID | 7040 |
| RTECS number | OP2275000 |
| UNII | 2611XJ14P6 |
| UN number | UN2662 |
| Properties | |
| Chemical formula | C7H8O2 |
| Molar mass | 124.14 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.129 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.25 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 10.1 |
| Basicity (pKb) | 11.54 |
| Magnetic susceptibility (χ) | -51.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.617 |
| Viscosity | 30.8 cP (25°C) |
| Dipole moment | 1.64 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 163.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -49.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3213.7 kJ/mol |
| Pharmacology | |
| ATC code | D11AX02 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 99°C |
| Autoignition temperature | 615 °C (1139 °F; 888 K) |
| Explosive limits | Explosive limits: 2.7–16% |
| Lethal dose or concentration | LD50 (oral, rat): 464 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (rat, oral) |
| REL (Recommended) | 0.0001 |
| IDLH (Immediate danger) | Not established |