Hydroquinone Monomethyl Ether, known as MEHQ or 4-methoxyphenol, has lived a colorful history since chemists in the late nineteenth century first laid out its structure. Originally born out of a search for better antioxidants, it soon found favor in polymer industries, laboratories, and medical research. People working in early plastics or photographic sectors quickly noticed its stabilizing effect, developing uses that stuck around for decades. Over time, research labs kept up the curiosity, tinkering with the molecule to answer bigger questions about oxidation and preservation. Today, it stands out as an example of how organic chemistry can create ripple effects across technology and modern industry.
MEHQ isn’t your everyday household name, but industries trust it for good reason. Chemically pinpointed as C7H8O2, it's a fine, pale crystal or powder, a bit aromatic in scent. The product keeps its cool in a bottle, warding off unwanted reactions in acrylic monomers, especially during storage and shipping. Those in charge of chemical batching find it mixes without fuss into resins, preserving quality like a vigilant night gardener chases away frost. From paints to pharmaceutical intermediates, reliable batches of MEHQ underpin the stuff people count on, from safety glass in cars to packaging keeping food fresh.
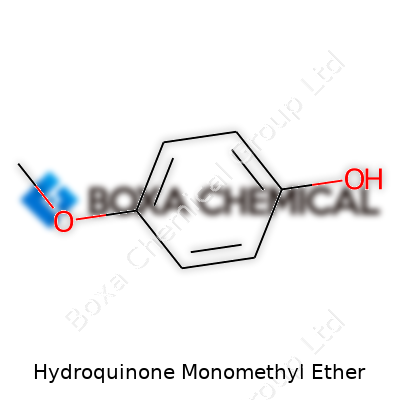
Hydroquinone Monomethyl Ether shows off its personality through physical traits. It usually appears as colorless to pale yellow crystals, melting between 54 and 56°C, with a boiling point close to 243°C. While it dissolves in ethers and alcohols, water handles it in lower doses. What sets it apart is the presence of a methoxy group, which tweaks its reactivity. It works double duty as a radical scavenger and antioxidant, holding up under stressful chemical environments where other compounds might falter.
Suppliers label MEHQ with meticulous detail—purity often runs north of 99%, impurities strictly tracked. Documentation walks buyers through CAS number (150-76-5), shelf life, safety instructions, and transport guidance. For anyone handling MEHQ in labs or storage depots, clarity on purity and allowed exposure levels means less guesswork and less risk. These details grow more critical as industries merge tighter quality controls with regulations for both workplace safety and downstream consumer security.
Production hinges on the methylation of hydroquinone. Standard syntheses use methanol as both reagent and solvent, where an alkali catalyst joins the party to transfer that all-important methyl group. The process takes careful timing; too slow and yields drop, too fast and purity takes a hit. Chemical plants invest in reactor systems to manage both heat and pressure, capturing nearly every drop of desired product while cutting out byproducts. Refined crystallization follows, then filtering and drying. The finished compound ends up packed for safe shipment, ready for diverse destinations.
MEHQ keeps chemists busy not only for what it can do, but what it can become. Its single methoxy group opens doors for further substitutions. In polymer chemistry, the molecule often intercepts free radicals, halting runaway chain reactions that otherwise spoil the batch. Labs often go further, putting MEHQ through reactions that build new pharmaceuticals, herbicides, and specialty chemicals. The ability to undergo etherification, oxidation, and coupling reactions gives it a reliable spot on the lab bench, always ready for new transformations.
In science, every compound seems to pick up a few names as it travels. Hydroquinone Monomethyl Ether answers to MEHQ, 4-Methoxyphenol, as well as p-Hydroxyanisole. In some circles, you’ll spot it tagged as p-Methoxyphenol or Monomethyl Ether of Hydroquinone. Chemical registries and industrial material safety data sheets rely on these tags to cut confusion, so anyone moving between suppliers or continents knows exactly what they are getting.
Working with MEHQ draws strict safety practices. Direct contact can irritate skin and eyes, inhalation triggers respiratory discomfort, and chronic mishandling only adds risk. Those who measure and mix it turn to goggles, gloves, local exhaust ventilation, and detailed training. Safety Data Sheets spell out exact hazard classifications, first aid measures, and storage requirements in no-nonsense language. Many plants dedicate isolated storage zones, preventing cross-contamination and accidental exposure. Safe transport depends on clearly labeled drums and robust packaging, so everyone down the chain remains protected.
MEHQ makes its mark across many sectors. Most notably, it preserves monomers used for acrylic glass—without it, runaway reactions could turn valuable material into useless lumps. Those manufacturing adhesives, coatings, and sealants rely on MEHQ to guard storage stability. In pharmaceuticals, it helps maintain shelf life of select drugs, while pigment and ink makers count on it to keep colors vibrant. Even in the food and cosmetic industries, carefully regulated use curbs oxidation and spoilage. Each application demands careful balancing: strong enough to block unwanted reactions, gentle enough not to interfere with product performance or safety.
Innovation teams rarely stop tinkering with MEHQ. Polymer chemists, looking for longer shelf lives and more sustainable materials, look for tweaks to the core structure. Downstream users demand purer, greener, or more effective formulations as consumer expectations grow sharper. Digital health and diagnostics have started eyeing MEHQ for roles in biosensing and advanced sample preservation. Robust research continues, integrating real-world data about performance, side effects, and alternative stabilizers. Open access to toxicity information and transparent supply chain tracking have become standard expectations for any supplier in the field.
MEHQ draws close scrutiny in toxicology circles, with dozens of studies testing its effects on humans, lab animals, and the environment. Short-term exposure tends to create mild irritation, but researchers have also mapped links between high concentrations and organ toxicity in animal models. Regulatory agencies around the globe weigh these findings, refining permissible exposure limits and workplace guidance accordingly. Wastewater and runoff studies highlight MEHQ breakdown under real-world conditions, an area still under study as more manufacturers seek to minimize environmental impact.
Sustainability trends keep pressure on chemical producers, prompting fresh strategies for safer production and more responsible use of MEHQ. Industry is already investing in closed-loop manufacturing and biodegradable polymers, where MEHQ can either play a key role or get replaced by next-generation stabilizers. Parallel work in green chemistry seeks milder, lower-energy synthesis methods, pushing for eco-labels and full lifecycle assessments. Future breakthroughs may soon bring new versions of hydroquinone ethers, purpose-built for digital fabrication, energy storage, or medical diagnostics. For now, MEHQ keeps securing its niche, proof that a small molecule can punch far above its weight in modern industry.
Hydroquinone monomethyl ether, often known as MEHQ, shows up in more places than most people expect. It looks like a powder or sometimes a colorless liquid and doesn’t carry a strong smell. Manufacturers turn to MEHQ mainly as a stabilizer, giving it work in areas where chemical chains could run out of control. Without it, products can spoil, turn yellow, or even become dangerous. For me, learning about this chemical pushed home how much modern life runs on things happening quietly in the background, often without much attention.
This compound earns its stripes in the plastics world. In polymer factories, the need to keep chemicals from reacting before they're supposed to always sits at the top of the engineer’s mind. MEHQ acts like a chaperone, making sure monomers—raw materials for making plastics—don't stick together too soon. Left unchecked, some acrylics and vinyls could react early, wrecking the batch. I once visited a facility where a faulty shipment skipped proper stabilization, and the result was thousands in lost product. Since then, safety sheets in those environments always list MEHQ as a critical ingredient.
Paints and coatings also count on MEHQ. People often expect these liquids to sit on a shelf for months before use. MEHQ slows down oxidation, letting products keep a smooth texture and consistent color. It helps keep nitrocellulose-based inks (the kind printers and publishers prefer) from clumping or changing color over time. A friend in the printing business once told me how a minor lapse in adding stabilizer led to entire pallets of ink going thick and unusable—an expensive lesson in why little ingredients like MEHQ make a big difference.
It’s not enough to just toss chemicals in and hope for the best. Health experts say working with MEHQ means respecting safety gear, good ventilation, and careful labeling. Direct contact can irritate skin or eyes, and breathing in its dust isn’t a good idea. Regulators like OSHA and agencies in Europe place strict guidelines on how much can end up in the air or water. Environmental groups worry about build-up in the ecosystem, so large users install filtration or use less hazardous alternatives when possible.
There’s an ongoing search for greener stabilizers. Some labs use phenolic antioxidants or less toxic quinones, but these haven’t replaced MEHQ everywhere yet. Engineers and chemists keep balancing safety, price, and performance. Sometimes, swapping to lower-toxicity chemicals means changing equipment, and that's a big investment. I’ve seen small companies partner with universities to test new options, trying to cut waste without sacrificing stability.
Most folks never think about stabilizers in daily goods, but they touch everything from cars and toys to the paint on walls. Chasing safer chemicals means healthier workers, cleaner air, and longer-lasting stuff. Learning where chemicals like MEHQ show up reminds me that modern life depends on unsung compounds—and that paying attention to what’s in our materials matters.
Hydroquinone monomethyl ether shows up in a lot of labs connected to rubber or plastic manufacturing. You might spot it under names like MeHQ or 4-methoxyphenol. Its job often involves keeping other chemicals from reacting too soon, acting as a stabilizer. When people ask about its safety, they might only think of basic personal protective equipment, but understanding the risks means looking deeper.
Anyone who has spent time working with industrial chemicals quickly sees that convenience and safety don’t always align. I remember an early morning spent in a poorly ventilated room where one small spill gave off an unpleasant sharp odor. Even though the chemical didn’t burn the skin instantly, letting it sit could easily lead to redness or dryness. Gloves and goggles aren’t for show—if skin or eyes come into contact, things get uncomfortable quickly. Inhaling the dust or vapor brought on irritation and a similar experience for colleagues. Nobody wants to get halfway through a day with itchy skin or a cough.
Scientific studies and workplace data point toward contact dermatitis for skin, eye irritation, and sometimes respiratory symptoms if airborne levels go unchecked. The National Institute for Occupational Safety and Health (NIOSH) includes MeHQ on its chemical hazard list, warning about long-term exposure risks. Chronic handling could lead to cumulative effects, the way that small cuts eventually scab over and leave a stubborn scar.
Toxicity data doesn’t raise as much alarm as some industrial chemicals, but there’s a pattern in the literature. Ingesting a significant amount can strain the kidneys and central nervous system. People accidentally exposed to high concentrations in factories have ended up with headaches or dizziness—symptoms that are easy to brush off, but add up over long shifts.
MSDS sheets recommend chemical-resistant gloves, tight-fitting eyewear, and fume hoods whenever possible. I’ve learned firsthand how important good ventilation is. Open windows and fans might not cut it for stronger odors or accidental splashes. Simple steps like changing gloves if they feel even slightly damp or never reusing lab coats make a huge difference in daily safety.
Factories and labs using larger volumes of MeHQ see most problems during cleanup and equipment maintenance. It’s easy to skip steps to save a few minutes, but over the years those shortcuts usually bite back. Thorough training, clear spill kits, and routines for regular air checks help avoid trouble before it starts. I’ve seen shops where workers remind each other about ventilation or gather once a month to review safety drills—it forges responsibility and keeps risks fresh in everybody’s mind.
People who work with chemicals like hydroquinone monomethyl ether aren’t looking for special treatment—just honest, useable information about risks and a culture that helps them stay safe long-term. Moving from basic hazard lists to sharing stories, practical tips, and learning from each other’s mistakes turns safety from a checklist into a habit.
For anyone with bottles of MeHQ on the shelf, active care matters. Reliable safety practices, up-to-date training, and a willingness to speak up about new concerns do more than limit accidents—they build trust within any lab or production line. In my experience, that’s the strongest kind of protection anyone can ask for.
Any chemist who’s spent time in a laboratory knows that some chemicals call for more respect than others. Hydroquinone Monomethyl Ether (also known as MeHQ) falls into that category. Used to prevent polymerization in monomers, it does its job quietly, but only if people give it the storage treatment it deserves. Ignoring its quirks means letting the chemical break down and potentially putting safety on the line.
MeHQ stabilizes more than just resins or monomers. It protects investments, workforce safety, and even research results. Once, during a summer internship, I watched a batch of expensive monomer take a nose-dive because someone stashed MeHQ at the wrong temperature. The lesson stuck. MeHQ wants a cool, dry home, and those factors affect not just its quality, but its ability to stop dangerous chain reactions.
Hydroquinone Monomethyl Ether’s packaging deserves attention. Opaque containers work best. Exposure to light can set off unwanted chemical changes—the type that not only ruin product quality but can lead to wasted inventory or worse, hazardous byproducts. Keeping it in a dark spot, away from direct sunlight, keeps it stable longer.
Temperature control matters too. MeHQ prefers to stay in the range of 15–25°C (roughly room temperature), away from sources of heat like steam lines or sun-soaked windows. High heat nudges reactions forward and degrades its effectiveness as an inhibitor. Cold is less troublesome, though extreme low temperatures can make the chemical more viscous and tough to work with.
Moisture introduces trouble. Any humidity that sneaks into storage can encourage clumping or even start unwanted side reactions. Desiccants inside drums, or dry storage rooms with managed humidity, keep the chemical fresh. Leaky seals or poorly fitted lids aren’t just minor annoyances—they’re invitations for spoilage.
MeHQ is flammable—never a detail to gloss over. Storage in a well-ventilated area reduces vapor buildup and helps control risks. Stashing stock near oxidizing agents or acids only amps up those risks. Segregating MeHQ in a flammables cabinet made me sleep easier while working in larger warehouses. Fire suppression measures, clear labeling, and up-to-date safety data sheets should go hand in hand with good storage practices.
Real stories shape better protocols. A misplaced container of MeHQ in a damp storeroom turned into a crystallized mess for my team once, and cleanup cost hours nobody wanted to waste. The fix was simple: regular checks for leaks and moisture, and a strict “no food or drink” rule near chemical storage. Staying on top of expiration dates and rotating stock means using the freshest supply first, preventing loss and unexpected downtime.
Good storage isn’t just about ticking boxes on a safety list. It means fewer headaches, steadier budgets, and a safer workplace. Any organization with MeHQ on the shelf stands to benefit from investing in staff training, clear signage, and a no-nonsense attitude toward housekeeping. In the long run, respect for storage sets the tone for respect throughout the lab or factory.
Hydroquinone Monomethyl Ether, sometimes called MEHQ or p-Methoxyphenol, brings its own set of properties to the table. Chemists, pharmacists, product handlers—they all keep a close eye on it because of how often it pops up in industries from cosmetics to plastics. Its chemical formula draws a lot of attention: C7H8O2. Knowing formulas doesn’t just satisfy a trivia itch—it helps people avoid mixing up similar-sounding compounds that might react differently, sometimes dangerously, under pressure or heat.
I’ve seen firsthand just how crucial it gets to be on point with chemical identifiers. It’s happened more than once that someone orders the wrong grade, or confuses compounds like hydroquinone (C6H6O2) and hydroquinone monomethyl ether (C7H8O2). The extra carbon, two extra hydrogens—these change how a chemical works, how it stabilizes other substances, or how safe it feels in your hands. In practice, the methyl group sitting on that oxygen gives MEHQ its “monomethyl” badge, offering it unique stabilization power, which polymerization industries put to good use.
MEHQ commonly shows up as an inhibitor in monomer storage tanks and shipping containers, slowing down unwanted reactions that lead to thick, unusable sludge. This property protects both the product and the people handling it. Mislabeling or mishandling hydroquinone compounds can easily cause costly accidents or legal headaches. I’ve read up on incidents where mixing up containers led to product waste and major regulatory fines. Chemical formulas act like a roadmap. If you misread it, you’re not only risking ruined batches but setting up health hazards—skin irritation, respiratory problems, or worse if things go sideways in a confined space.
Publications from the U.S. National Library of Medicine detail how p-methoxyphenol stabilizes products like acrylic paints and adhesives. The Centers for Disease Control and Prevention list it under substances needing clear labeling in workplace safety plans. Regulatory frameworks in Europe, Asia, and across North America echo the same point: the right formula makes for a safer workplace and a clearer audit trail.
It pays off to build routines checking and double-checking chemical labels. Using digital inventory systems, barcodes, and easy-access databases cuts down on errors. Training up new staff or colleagues should always include a focus on recognizing formulas, not just the names. If a bottle reads C7H8O2, it’s hydroquinone monomethyl ether; anything off means double-check before you move it to the next step.
At a time when oversight agencies step up surprise inspections and fines, accuracy has real consequences. It’s tempting to skimp on details, but with enough reminders and education, that temptation runs out of steam. One wrong character in a formula has cost companies not just money, but their reputation and workers’ health. Take the time to lock in the basics—chemical formulas like C7H8O2 need to be front and center, not buried in paperwork.
Hydroquinone Monomethyl Ether isn’t a chemical anybody wants to mess up with. Used often in stabilizing other reactive substances, it can slip into labs or manufacturing settings where it quietly waits for someone to forget just how hazardous it can get. Tossing this into the basic waste stream might seem tempting, but this route often spells trouble for both people and the environment. If personal experience in a shared research lab taught me anything, it’s that small mistakes in chemical disposal can turn bad fast—one spill, and you’re scrambling for the right neutralizer, anxious about inhaling fumes or causing an accident. That worry extends far beyond the lab: improper disposal means toxins leaching into soil or waterways, and nobody wants to fish their dinner out of a polluted lake.
Chemicals like Hydroquinone Monomethyl Ether love to interact with other substances, and not always in friendly ways. Direct dumping straight into drains or trash bins can expose sanitation workers or create toxic byproducts. According to the U.S. Environmental Protection Agency, chemicals with phenolic compounds, like this one, have shown up in ground and drinking water—cases tied directly to careless disposal. People buy bottled water or worry about where their tap water comes from, often missing the quiet failures in hazardous waste handling. One wrong move, and small communities end up with tough questions no average person should have to field. Years ago, a colleague found herself sick after an unmarked bottle turned up in regular trash; tracing it back became a lesson nobody forgot.
I’ve learned that it’s smarter—and safer—to follow scripted chemical protocols, even when shortcuts look tempting. Hydroquinone Monomethyl Ether belongs to specially controlled waste bags or containers clearly labeled as hazardous organic waste. Never mix it with other solid or liquid refuse. For those in workplace settings, chemical hygiene plans usually spell out process flows, and skipping these steps isn’t just risky—it’s illegal. The Occupational Safety and Health Administration holds facilities to standards that keep both workers and the outside world safe. In a home lab or smaller operation, hazardous materials collection events can take dangerous leftovers off your hands. Most cities sponsor these annually, where folks show up with odd bottles and go home lighter and relieved. It sounds simple, but until these services became regular in my area, most folks simply guessed or hoped their methods were good enough. That’s a gamble you regret once the results turn up in someone’s well water.
Technology hasn’t solved disposal for us. Containerizing waste for licensed hazardous waste handlers costs money, but every cent avoids thousands in environmental restoration or medical bills. Staff training makes a massive difference: a five-minute refresher can cut disposal mistakes dramatically. Manufacturers can ship small quantities with return labels, sparing users from tough decisions about leftovers. Communities succeed when government, industry, and private citizens put their heads together—funding waste pickups, staffing hotlines for chemical questions, and teaching new generations the cost of ignorance.
The choices people make with destructive chemicals like Hydroquinone Monomethyl Ether ripple outward. Genuine care for other people’s health and the world around us starts with simple steps—clear labels, smart storage, and always opting for a safe, legal disposal route. Everyone doing a bit more reflection and following established processes ends up with cleaner hands and cleaner water for all.
| Names | |
| Preferred IUPAC name | 4-Methoxyphenol |
| Pronunciation | /haɪˌdrɒkwɪˈnoʊn ˌmɒnoʊˈmɛθəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 100-66-3 |
| Beilstein Reference | 1306396 |
| ChEBI | CHEBI:24681 |
| ChEMBL | CHEMBL165788 |
| ChemSpider | 12990 |
| DrugBank | DB14004 |
| ECHA InfoCard | 100.011.523 |
| EC Number | 205-769-8 |
| Gmelin Reference | 8416 |
| KEGG | C01737 |
| MeSH | D06.472.363.340.350 |
| PubChem CID | 7058 |
| RTECS number | OA2450000 |
| UNII | 7P9PR63G5T |
| UN number | UN3076 |
| CompTox Dashboard (EPA) | DTXSID2020140 |
| Properties | |
| Chemical formula | C7H8O2 |
| Molar mass | Molar mass: 124.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Faint aromatic odor |
| Density | 1.107 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.61 |
| Vapor pressure | 0.67 mmHg (at 25 °C) |
| Acidity (pKa) | 11.25 |
| Basicity (pKb) | 8.04 |
| Magnetic susceptibility (χ) | -44.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.538 |
| Viscosity | 8.6 mPa·s (25 °C) |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 137.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -225.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3227 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D03AX02 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H312, H315, H319, H332, H341, H373 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P370+P378, P403+P235 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 79°C |
| Autoignition temperature | 287°C |
| Explosive limits | Explosive limits: 2.5–27.6% |
| Lethal dose or concentration | LD50 (oral, rat): 1,270 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1,430 mg/kg |
| NIOSH | NIOSH: OP4550000 |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | Not listed. |