The story of Hydroquinone Dimethyl Ether tracks a long trail through organic chemistry labs, beginning over a century ago. Early researchers, busy exploring ethers and their use in dyes and pharmaceuticals, landed on this compound as a logical extension of their work with hydroquinone. As chemical manufacturing matured, these discoveries picked up pace, and processes kept evolving. In the post-war era, with demand for advanced polymers and antioxidants climbing, the value of Hydroquinone Dimethyl Ether did not escape sharp-eyed analysts and chemists. Growing markets in the twentieth century pulled this molecule out of dusty journals and into high-volume production, especially as industries searched for stabilizers in wire insulation, adhesives, and paints.
Hydroquinone Dimethyl Ether steps into the spotlight with a proud record across sectors. Known among chemists as 1,4-Dimethoxybenzene or p-Dimethoxybenzene, the compound’s roots tie strongly to the production of specialty chemicals and fine intermediates. Factories stockpile it in drums or bags, ready for both custom synthesis and as a stabilizer that firms up other products. Its sharp, slightly sweet odor gets noticed when opening storage containers, making it easy to identify in a busy warehouse. Purity levels push above 99% with standard grades heading to electronics or pharma partners, where small contaminants bring lots of trouble down the line.
With a melting point near 54°C and a boiling point hitting over 170°C, Hydroquinone Dimethyl Ether moves between solid and liquid states with the flick of a thermostat. Chemically, this aromatic ether resists attack from weak acids and bases but gives way in the face of strong oxidizers. The molecule packs two methoxy groups on the benzene ring, setting it apart from plain hydroquinone. Those subtle differences deliver big changes; it slides easily into organic solvents like ether or acetone but shrugs off water. Handling this material means keeping an eye on flammability. As a crystalline solid, it dusts quickly if poured too fast, calling for gloves and a respirator. Left in open air, light and heat will start slowly degrading its purity, so careful packaging remains key to shelf life.
Suppliers stamp Hydroquinone Dimethyl Ether with batch numbers, production dates, purity percentages, and hazard pictograms. Safety Data Sheets give a rundown of flash point (around 83°C) and storage advice. Labels match globally harmonized standards, noting environmental hazards and first aid rules. Industry buyers expect transparent reporting on synthetic byproducts and heavy metals, knowing regulators audit these records in every region. For process engineers, the most valuable spec often turns out to be particle size and flow; inconsistent granules can clog automated feeders during continuous production, wrecking efficiency and output. Barcode labeling now helps factories track inventory and reduces mix-ups in multi-compound facilities.
Commercial production of Hydroquinone Dimethyl Ether usually relies on methylation of hydroquinone, most often with dimethyl sulfate or methyl iodide in the presence of a base like potassium carbonate. The reaction runs best under anhydrous conditions to avoid unwanted side reactions. As a process chemist, I once found that a small jump in temp knocks product yield off target, so operators monitor probes and jacket temperatures relentlessly. Purification steps include solvent extraction, washing, and re-crystallization to squeeze out remaining unreacted starting materials. Waste disposal practices now focus on spent methylating agents—watchdogs regulate emissions, and closed systems keep operator exposures low.
Hydroquinone Dimethyl Ether plays well as a starting point for downstream synthesis. Its methoxy groups lay the foundation for nucleophilic substitution, demethylation, and aromatic ring reactions. For instance, many colorant manufacturers use it to prepare azo dyes, while in pharma, it often gives rise to complex ring systems used as intermediates for active ingredients. In the specialty polymers field, this molecule becomes a cross-linker or a chain terminator, letting manufacturers dial in material properties. From personal trial, slow addition of oxidants keeps runaway reactions in check—one slip and you end up with tars clogging the reactor, showing every step needs hands-on attention.
Known by a handful of aliases, including p-Dimethoxybenzene, 1,4-Dimethoxybenzene, and Hydroquinone Methyl Ether, the compound also appears under trade names, depending on supplier. Research catalogs sometimes call it quinol dimethyl ether, though most industry buyers stick to IUPAC convention. Regulatory filings demand tight documentation of synonyms, bringing clarity to customs officials and compliance auditors alike, where misunderstanding can delay deliveries at border controls. Some suppliers use custom brand names for high-purity grades, aiming to carve out space in electronics, pigments, or pharmaceutical sectors.
Tough safety cultures develop where Hydroquinone Dimethyl Ether gets used on a large scale. Spills call for evacuated areas and quick-action absorbent materials—routine drills show newcomers how fast small leaks spread vapors in a busy plant. Fire marshals insist on grounding containers during transfers to prevent static discharge. Line operators wear gloves, goggles, and flame-resistant gear for routine handling. Technicians train with respirators since dust or vapor exposure can spark headaches and respiratory irritation. Waste streams run through dedicated collection tanks before incineration, monitored by on-site environmental techs who run spot checks, knowing non-compliance penalties stack up fast.
Industries put Hydroquinone Dimethyl Ether to work in many roles. It acts as a stabilizer for resins, shielding them from heat or light-induced breakdown. Its key spot in dye and pigment manufacturing comes from its reactivity and stability. Electronics makers value its use as a building block for organic semiconductors and as a component in photoresist chemicals—every tiny flaw in batch purity can spoil screens or chips worth millions. The compound shows up in antioxidant blends for rubber and polymer products, limiting aging and extending shelf life. Research labs count on its reliability when working with complex organic syntheses, trusting in its consistency to provide predictable results time after time.
Lab teams all over the world keep searching for new catalysts and reaction conditions that cut costs or boost yields for Hydroquinone Dimethyl Ether. Some academic groups collaborate with chemical giants to develop greener methylating reagents, aiming to dump traditional toxins like methyl iodide and dimethyl sulfate for safer alternatives. Startups experiment with flow reactors, achieving better temperature control and leaner energy use compared to old batch setups. Researchers track product stability during long-term storage, monitoring yellowing or impurity drift as climate-controlled warehouses guard against hot summers and frigid winters. Conference presentations spark surprise at new applications—just last year, a team showed that small tweaks to the synthesis route led to pharmaceutical precursors with improved performance.
No industrial compound enters wide use without toxicity scrutiny, and Hydroquinone Dimethyl Ether holds no exception. Studies in rats and mice exposed to high doses reveal moderate acute toxicity but no strong evidence of carcinogenic risk at routine occupational exposures. Lab testing points to skin and eye irritation, so field guidelines mandate gloves and splash shields. Chronic exposure data stay limited; regulatory bodies call for ongoing animal testing and tighter air monitoring at large sites. Wastewater analysis tracks metabolite breakdown to ensure municipal treatment works can handle discharge, with any unexpected bioaccumulation flagged for further study. Workers trust management to keep air and liquid controls tight, knowing personal stories from decades past where safety took a back seat to production numbers.
New green chemistry frameworks push for more sustainable production methods. Companies hesitate to adopt methods using hazardous methylating agents, opening the door for bio-catalytic or solid-acid alternatives. Analytical chemists develop better impurity tracking, leaning on high-resolution mass spectrometry, which stretches performance boundaries for electronics and pharmaceutical applications. The electronics sector keeps growing, fueling demand for high-purity grades as screens and chips become more critical to daily life. Pressure from downstream users means suppliers work harder to cut heavy metals and unwanted byproducts. As regulatory standards grow stricter, old plants retrofit their gear, and new setups spring up in expanding industrial parks across Asia and the Americas. Researchers keep laying out ambitious goals for combining productivity with worker safety, tracking every new advance in lab journals and industry reports.
Hydroquinone dimethyl ether stands out in chemical manufacturing, especially in how companies fight off oxidation. Since oxidation can wreck products—changing color, reducing shelf life, sometimes even creating unexpected hazards—chemists look for solid antioxidants. This compound becomes a go-to choice in a variety of formulas, from industrial greases to specialty polymers. Working in research, I’ve grabbed for this chemical to help certain plastics last longer under exposure to air or light. Without it, materials would yellow, crack, or just break down way faster.
Plastic and rubber manufacturers lean on hydroquinone dimethyl ether during polymerization. Stick a batch of styrene or butadiene in a reactor, and there’s a risk things will run out of control. Unchecked heat and runaway reactions can turn a plant upside down. This stabilizer acts as a safeguard. It helps keep the production lines stable and the final products resilient. For companies making tires, hoses, or even medical tubing, that stability can mean fewer recalls and less waste.
Rubber does not do well on its own fighting off the aging effects from sun, oxygen, or daily wear. Mix in this ether, and the material’s structure can stand up longer. Factories see fewer defects, and end users find their goods keep working as promised. Knowing that the same chemical keeps both a car tire and an IV line dependable gives it real relevance.
Hydroquinone dimethyl ether shows up in cosmetics, mostly as a stabilizer. For lotions and creams with delicate fragrances or plant extracts, unwanted chemical changes can spoil both safety and scent. Adding a reliable stabilizer helps those products stay fresh on the shelf. Having worked with formulators, I’ve seen how crucial this is for smaller companies aiming to avoid angry calls from shoppers over spoiled creams.
With all its benefits, mistakes happen if the chemical isn’t managed right. There’s always discussion about safe levels in personal care and consumer products. Some experts debate its link to health issues, so regulatory agencies in the US and Europe have stepped in with strict guidelines. Manufacturers should keep detailed records, train staff in safe handling, and regularly test for any breakdown products. My years in a lab showed just how fast a slip in safety can lead to headaches for both companies and everyday people.
Demand keeps rising for chemicals that do the job but bring less baggage. Researchers are screening safer, plant-derived antioxidants. Change happens slowly because many alternatives don’t match hydroquinone dimethyl ether’s price or performance yet. Some companies focus on making cleaner production and better disposal techniques to lower risks—even when the chemicals themselves remain the same.
For the foreseeable future, hydroquinone dimethyl ether continues supporting industries we depend on, from car tires to everyday creams. Careful research, updated practices, and honest communication with customers can help make its benefits count while minimizing the risks.
People look for brighter, more even-toned skin all the time, and skin lightening products pop up everywhere. Hydroquinone has a big reputation among these products, especially for treating hyperpigmentation like dark spots, melasma, and freckles. Some new formulas swap regular hydroquinone for an ingredient called Hydroquinone Dimethyl Ether (HDE), hoping for fewer side effects. The question is, can these promises be trusted?
Regular hydroquinone has drawn a lot of attention because of both its results and safety concerns. Chronic use can trigger something called ochronosis—bluish-black discoloration, especially in darker skin types. Some places even restrict or ban over-the-counter sales. HDE steps in as a supposed gentler alternative, touted for similar brightening effects with less irritation.
Known in the lab as 4-methoxyphenol, HDE shares a chemical family line with hydroquinone but adds a methoxy group, hoping to cut down toxicity. Early studies say HDE shows less cytotoxicity in isolated cells. Dermatologists I’ve spoken with call it promising, but say limited use data keeps them cautious. I know a couple of people who tried serums containing HDE—patch test on the inner arm went fine, but small breakouts still popped up when used for longer than a week.
Looking to the published research, you will not find large randomized clinical studies on HDE. Most safety claims come from small trials and unpublished company data. By contrast, the typical skincare ingredient that earns dermatologist trust has survived decades of independent trials plus government safety review. So far, HDE lacks that kind of track record.
Some brands suggest HDE works as a “safer” alternative for people who react badly to regular hydroquinone. Lower skin irritation levels mean less redness, flaking, and burning—a huge plus for people sensitive to stronger formulas. The cosmetic industry counts on consumer desire for non-prescription solutions, and HDE fits the marketing bill neatly. People tired of waiting for a prescription might feel tempted to try newly labeled “gentle brightening” products.
This leads to risks of self-experimentation. Most doctors I know tell patients, “If you’re eager to fade pigment spots, know exactly what’s in the formula.” The packaging doesn’t always explain the real risks. Long-term data for HDE is missing. Nobody knows exactly how repeated use changes skin in five years, especially for people from different ethnic backgrounds or with varying health conditions.
Regulators and cosmetic manufacturers share responsibility here. Proper labeling, batch safety testing, and open ingredient lists help consumers sort facts from flashy promises. Dermatologists should get up-to-date safety sheets so they’re ready when questions about HDE surface in the office. For now, anyone drawn to try HDE should spot-test, check with a board-certified expert, and keep an eye out for evidence from reputable journals.
Following evidence over hype makes sense for everyone, especially with any new skin ingredient. Real safety comes from transparency, not trendiness. If HDE lives up to claims with future scientific backup, it deserves a spot in people’s skin routine. Until then, a healthy dose of skepticism—plus backing every new step with expert guidance—serves every skin type best.
People search for ways to get rid of stubborn dark spots, uneven skin tone, or pigmentation. Hydroquinone dimethyl ether pops up in a lot of skin-lightening products as a chemical cousin to traditional hydroquinone. Many turn to it hoping for fast and visible results, but not enough talk happens about what might come with the territory. Safety gets lost in the quest for brighter skin.
Plenty of users report redness, itching, and burning—not exactly surprises for anyone who's tried harsh skincare. Over time, I’ve watched friends give up on skin lighteners after a rash or stinging that just won’t calm down. Dermatologists warn patients about allergic reactions, too. Flaking and peeling pop up, especially if you have sensitive skin. The urge to scratch can make things worse, turning a small patch into a much bigger problem.
I’ve noticed people think using a bit more cream or applying it more often speeds up results. What they do not realize is that repeated and long-term use leads to something called ochronosis—bluish-black skin darkening that’s tough to reverse. Reports from both Africa and Asia show that misuse leaves permanent marks. A study in the International Journal of Dermatology points to a rise in this condition in communities who use skin lighteners without guidance from professionals. Doctors see this more in over-the-counter creams where users don’t always read warnings or follow directions.
Most side effects stay on the skin’s surface, but that’s not a guarantee. Some data suggest that chemicals like hydroquinone dimethyl ether could get absorbed if the skin barrier breaks down. Once in the body, they can cause headaches, nausea, or mild dizziness. Evidence remains limited, and researchers continue to study how much actually reaches deeper tissues. Yet, for those with pre-existing liver or kidney problems, any added risk raises concern.
The controversy gets bigger with talk about what happens inside cells. Some lab studies show these compounds interfering with melanin production and possibly disrupting hormone activity. No proof yet exists that hydroquinone dimethyl ether causes cancer, but other similar chemicals have come under scrutiny. Many countries, including those in the European Union, limit or ban its use in cosmetics out of caution. Regulatory bodies in Australia and Japan followed suit, pulling products from shelves based on safety evidence.
Reliable sources recommend using any skin-brightening ingredient only with guidance from a certified dermatologist. My own experience echoes this: Trying to tackle pigmentation at home brings more stress than success. Safe alternatives—like vitamin C, azelaic acid, or even simple sunscreen—help over time without as many risks. Broad-spectrum sunscreens limit sun damage and prevent new spots before they start. If you ever spot new symptoms or worsening irritation, seeking medical advice beats waiting for things to settle down. Reading product labels, asking about the source, and sticking to trusted brands help keep things in check as well.
Chemicals like hydroquinone dimethyl ether show up in many industries, especially labs and manufacturing where careful handling makes all the difference. After years spent working with compounds that lose their punch or even turn dangerous outside of best storage practices, I've seen mistakes that cost time, money, and sometimes safety. Proper storage protects not just the shelf life but the people handling it every day.
Hydroquinone dimethyl ether reacts fast to air and light. Left exposed, it tends to darken—a pretty clear sign of breakdown. This isn’t about looks. The compound loses purity, changing how it behaves in experiments or production lines. Labs usually pick amber glass bottles for a reason. These containers keep light out, and tight-fitting lids cut down on contact with air.
A study from a specialty chemicals journal once showed degradation rates climb as temperature rises. Anything above room temperature means shorter shelf-life. Years in a small university lab taught me to rely on the lower shelves of chemical fridges. Temperatures between 2°C and 8°C fit the bill, and the fridge needs constant monitoring. High humidity can make a mess by introducing unwanted water into the bottle.
Water speeds up the breakdown process and can set off unwanted side reactions. I learned the hard way that just dropping a desiccant pack in storage cabinets keeps everything much drier. It also helps to clearly label the bottle with the opening date and storage details, since memory sometimes fails and nobody enjoys guessing whether a chemical’s still good.
Lab benches might seem handy, but crowded shelves and hasty hands spill chemicals more often than most admit. Dedicated flammable cabinets built to vent fumes keep the storage area safer. Make sure surfaces under the bottles resist corrosion—hydroquinone dimethyl ether may not bite like acids, but spilled liquids still cause headaches over time.
Dirty spatulas or pipettes taint a stock bottle fast. Fresh gloves, cleaned glassware, and strict attention during dispensing save future batches from invisible problems. Every time I skipped this step, strange results or off odors crept up weeks later.
Good storage starts with strong labeling. Permanent marker often fades in chemical cabinets. Printed, water-resistant labels stick through handling and the occasional spill. Mark down the name, hazard level, concentration, storage date, and storage conditions. My old habit of scribbling initials on lids kept coworkers honest, too. Everyone could trace mishandling back and fix training gaps.
Safe chemical storage blends science with plain vigilance. Replace loose stoppers right away, check for leaks, and move expired or suspect materials to waste. Add regular reminders to staff meetings. The risk of one mistake might seem far away, but experience—and a look at regulatory fines—proves otherwise. Good habits are easier to keep than to build, and smart storage safeguards both products and people.
A routine of temperature checks, record-keeping, and physical tidiness shapes better outcomes. With chemicals like hydroquinone dimethyl ether, attention to detail saves more than time: it protects health and supports real trust in the results being produced from every bottle.
Stories about skin-lightening products tend to spark debate. Hydroquinone Dimethyl Ether is catching attention lately, with buyers in stores and online suddenly wondering if a doctor’s note is necessary to grab a tube. There’s a good reason for the confusion. Skin care shelves are full of dazzling claims, but this one isn’t just another moisturizer; Hydroquinone derivatives can change how skin looks in dramatic ways, often chasing away dark spots—melasma or the tougher pigment left from acne scars.
The heart of the matter boils down to safety. Hydroquinone itself isn’t a gentle ingredient. Over-the-counter strength sits at 2%, but stronger stuff—enough to really bleach the skin—usually lands behind the pharmacy counter. Some countries, including the U.S., draw a sharp line: prescription only for higher doses. Asia and Africa have sometimes seen looser rules, even for chemical cousins like Hydroquinone Dimethyl Ether, but regulators keep looking closer because reports of skin damage and long-term health worries surface in medical journals every year.
Ask any dermatologist about hydroquinone, and the word “caution” instantly crops up. Years back, I tried a skin-lightening product bought off the shelf. It faded my freckles, but after two weeks my cheeks started peeling, and little bumps broke out. The box never mentioned what to watch out for or when to stop. Not everyone reacts the same way, but stories like mine aren’t rare. Too much hydroquinone—or using it over months—can cause ochronosis, a bluish-black discoloration that sometimes won’t fade. Some studies have also raised questions about systemic absorption and cancer risk, especially if used with little oversight.
That’s where prescriptions come in handy. Doctors keep tabs on side effects. They check for signs of overuse or improper application and keep people informed about alternatives. The Food and Drug Administration and the European Medicines Agency track these risks closely. In the past decade, they’ve started pulling certain products off shelves or making the prescription label mandatory.
People want quick fixes for stubborn skin spots. Being able to order hydroquinone dimethyl ether online seems like a blessing. No waiting rooms, no awkward questions, just fast relief. But this easy access opens the door to overuse, misuse, and sometimes dangerous mixing with other harsh chemicals. Sometimes people layer products, hoping to speed up results, and end up with permanent damage or allergies doctors struggle to reverse.
Online pharmacies and beauty shops don’t always play by the rules. Many sell products in strengths or doses far above what’s considered safe, and labels can be misleading or downright incorrect. The push for beautiful, flawless skin pressures people into risky choices. I’ve seen friends order creams with little knowledge of what’s inside—no dermatologist in the loop, and no way to confirm the source’s reliability.
There’s a smarter way to approach this. Prescription rules protect more than just law and order. They safeguard users from hidden dangers. Strong ingredients deserve respect—a health professional’s oversight brings that balance. For anyone worried about dark spots, talking things through with a dermatologist helps make sense of the options, filters out myths, and steers you toward safer, more sustainable results.
At the end of the day, clear rules and transparent labeling help win trust. Tighter controls on hydroquinone dimethyl ether benefit users, not just regulators. Getting good advice shouldn’t feel like a hurdle, especially where health and confidence intersect.
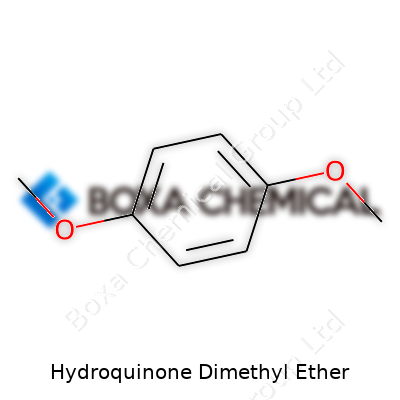
| Names | |
| Preferred IUPAC name | 1,4-Dimethoxybenzene |
| Pronunciation | /haɪˌdroʊkwɪˈnoʊn daɪˈmɛθɪl ˈiːθər/ |
| Identifiers | |
| CAS Number | **150-76-5** |
| Beilstein Reference | 1635480 |
| ChEBI | CHEBI:28521 |
| ChEMBL | CHEMBL430668 |
| ChemSpider | 8278 |
| DrugBank | DB14020 |
| ECHA InfoCard | 69a64561-e45e-4ce8-8a1e-9ce126a7a438 |
| EC Number | 204-924-6 |
| Gmelin Reference | Gmelin 82579 |
| KEGG | C01533 |
| MeSH | D016696 |
| PubChem CID | 8055 |
| RTECS number | MN1575000 |
| UNII | V5A4UJ88GP |
| UN number | UN2664 |
| Properties | |
| Chemical formula | C8H10O2 |
| Molar mass | 154.18 g/mol |
| Appearance | White crystalline powder |
| Odor | Characteristic |
| Density | 1.12 g/cm3 |
| Solubility in water | Insoluble |
| log P | 0.88 |
| Vapor pressure | 0.24 mmHg (at 25°C) |
| Acidity (pKa) | 19.24 |
| Basicity (pKb) | 7.86 |
| Magnetic susceptibility (χ) | -59.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.538 |
| Viscosity | 2.24 mPa·s (25 °C) |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 122.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -293.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2909 kJ/mol |
| Pharmacology | |
| ATC code | D11AX02 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. May cause an allergic skin reaction. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 87 °C |
| Autoignition temperature | 540°C |
| Explosive limits | Upper: 8% Lower: 1.6% |
| Lethal dose or concentration | LD50 (oral, rat): 550 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 3710 mg/kg |
| NIOSH | WZ4725000 |