Picture the tail end of the 20th century—researchers started to eye advanced phenolic antioxidants as industries began pushing for better protection against oxidation, especially for plastics and fuels. Chemists needed something with muscle, so attention swung to substituted xylenols. 6-Tert-Butyl-2,4-Xylenol emerged from decades of organic chemistry, with researchers looking for molecules tough enough to disrupt oxidation chains without falling apart under heat or light. This compound didn’t just pop up; it grew from steady progress in phenol chemistry and from recognizing how small tweaks in structure—like a tert-butyl group at just the right spot—change a molecule’s performance and safety profile. Today’s widespread industrial interest has roots in trial and error across labs in Europe, the US, and Asia, always chasing better antioxidants for ever-evolving polymers and fuels.
There’s nothing mysterious about why industries keep turning to 6-Tert-Butyl-2,4-Xylenol. Its track record in protecting synthetic materials and fuels makes it a real workhorse. This compound isn’t limited to obscure applications; it works its way into paints, adhesives, lubricants, and even personal care products. A lot of scientists and engineers have relied on its ability to prevent the slow breakdown of materials that cost companies millions through product failures or recalls. Companies don’t just buy any antioxidant—they look for something that won’t deliver nasty surprises in formulation stability, shelf life, or material performance.
6-Tert-Butyl-2,4-Xylenol comes as a white, crystalline solid. The compound holds together under heat better than many phenols, sticking around past 125°C before melting. Its smell isn’t overpowering, but it has that sharp, antiseptic aroma common to phenols. Thanks to the tert-butyl group, it cuts down on volatility and slows the rate at which it reacts with oxygen in the air. Solubility isn’t universal; it brings decent solubility in organic solvents such as ethanol, ether, and chloroform, but water—like with most phenols—is not its friend. In practice, this means formulators can get it into systems where water isn’t dominant, like plastics and fuels.
Manufacturers and buyers usually pay close attention to purity, with specifications demanding at least 98% assay by weight. UV absorption and thin-layer chromatography help verify it’s no impostor or mixture. Labels indicate its chemical name, a range of synonyms, and safety data—flammability, storage instructions, and warnings about contact. Container integrity matters too because moisture degrades the compound faster than most realize. Those who try to skirt proper storage often face product loss, not to mention surprise compliance headaches if documentation fails to line up with what agencies like OSHA need.
Making 6-Tert-Butyl-2,4-Xylenol typically starts with the alkylation of 2,4-xylenol using isobutylene or tert-butyl alcohol with an acid catalyst, such as sulfuric acid or an ion-exchange resin. Careful temperature control is non-negotiable here, as high heat can push things toward unwanted side products and reduce yields. After reaction, standard work-up steps—neutralization, extraction, and recrystallization—help isolate the product at high purity. Small lab setups and full-scale chemical plants both stick to this basic recipe because it minimizes byproducts and sets up the right product without expensive purification steps—a lesson learned over decades of process optimization.
The phenolic hydroxyl of 6-Tert-Butyl-2,4-Xylenol opens the door to plenty of modifications. For example, chemists can etherify that group for more lipophilic antioxidants, or they’ll use acylation to tune solubility profiles for particular resins or oils. Oxidation reactions are possible but less favored since the tert-butyl group, bulky and electron-donating, offers strong protection, making oxidation sluggish. For material scientists, these properties are gold because stable antioxidants outlast others in polymers and rubbers. Many researchers tinker with the aromatic ring or substitute halogens or alkyl groups to push boundaries on performance or environmental persistence.
A quick search shows this compound under a handful of trade names and synonyms. Some call it TBX, others use systematic International Union of Pure and Applied Chemistry designations. On labels and safety sheets, expect to see “2,4-dimethyl-6-(1,1-dimethylethyl)phenol,” “tert-butyl-xylenol,” or the casual abbreviation “6-t-butyl-2,4-xylenol.” Sometimes older literature calls it “antioxidant BHT” by mistake, but that points to the necessity of thorough documentation at all levels—no one enjoys a mix up that compromises a manufacturing process.
Workers and researchers run tight ship with phenols, and 6-Tert-Butyl-2,4-Xylenol calls for the same respect. Skin and eye protection isn’t just a formality—this compound irritates fast and can cause long-lasting discomfort. Ventilation is a must where dust or fumes might accumulate during handling or transfer. Regulatory bodies demand detailed hazard labeling, and companies set strict exposure limits in line with local statutes and international guidance. More companies now lean toward automated transfer systems, cutting down on human error and direct contact. Spill response relies on standard phenol protocols—absorb with inert materials, avoid environmental discharge, and never mix with acidic or basic waste streams.
Industries leverage 6-Tert-Butyl-2,4-Xylenol where thermal stability and effective radical scavenging set the benchmark for antioxidants. Plastics manufacturers use it to extend the service life of polyethylene, polypropylene, and styrenic resins. In fuels and lubricants, it stops gums and varnishes from forming under storage or extreme pressure. Paint and coating suppliers rely on it for color preservation and elasticity over long shipping routes and shelf times. Some cosmetic formulas, especially those with volatile or precious oils, include small amounts to block oxidation. Electronics firms—always hungry for reliable insulators—tap its use in high-grade coatings and potting materials where long-term durability makes or breaks a product line.
Academic groups and industrial labs probe new frontiers for phenolic antioxidants almost daily. Ongoing work focuses on tuning the molecular backbone to maximize performance while reducing toxicity and environmental footprint. Some teams explore blends with other antioxidants to create synergistic effects—results point to improved performance even at lower doses. There’s also a drive to understand degradation mechanisms, using real-time analysis and computer modeling to predict lifetimes in polymers, lubricants, and even medicine. Funding and patent data reveal a steady uptick in interest, especially as manufacturing trends push towards thinner, lighter, and more demanding synthetic materials.
Toxicologists approach phenols with caution. Research finds that 6-Tert-Butyl-2,4-Xylenol has moderate acute toxicity; it disrupts cell membranes and can irritate skin and eyes. Some animal studies point to liver and kidney stress at very high doses, raising flags for long-term exposure. Regulatory agencies scrutinize these findings, guiding manufacturers to limit human contact in downstream products and enforce proper handling in plants. Companies perform routine monitoring—bloodwork and environmental sampling—for those working with raw phenols for extended periods. Safe disposal practices prevent the compound from accumulating in water supplies or soils, where it could cause more trouble for populations far from the original site.
Looking ahead, demand for materials resilient to oxidation and thermal stress will keep 6-Tert-Butyl-2,4-Xylenol and its cousins relevant. Regulations tighten each year on toxicity and environmental safety, so manufacturers lean on research to deliver safer, biodegradable replacements. Advances in synthesis could lower costs and shrink the environmental impact of production, while digital tools help design tailored molecules faster than ever before. There’s also growing collaboration between academia and industry, with teams pushing for greener production methods and innovative uses in batteries, catalysis, and even medicine. The same push that brought this compound to market decades ago—the ongoing search for robust, safe, and sustainable materials—ensures that researchers and companies keep raising the bar.
After working with old cars in my uncle’s garage, I started paying more attention to the stuff we put in engines and cleaning products. Science often hides behind long names like 6-Tert-Butyl-2,4-Xylenol, but this compound pops up more than most folks expect. It helps oils, fuels, plastics, and cleaners last longer and work better. Stripping away the chemical jargon reveals a compound at the crux of keeping rust or mold from ruining the things we use in daily life.
Plastics crack and fuel thickens when oxygen does its thing. Using 6-Tert-Butyl-2,4-Xylenol, known to chemists as an antioxidant, firms up the lifespan of these products. Tossing plastic wrappers or cleaning wipes into a storage cabinet means trusting they won’t degrade or turn useless. This chemical shields polymers in plastic and synthetic rubber, stopping the slow breakdown that sunlight and air cause. In the world of lubricants, lube oil for engines benefits, as engines run hot and invite breakdown. The antioxidant blocks the chains of oxidation, holding back sludge and gunk that could bring a machine to a halt.
Hospitals and supermarkets both fight the same enemy—germs. Cleaning sprays and antiseptics lean on ingredients that kill bacteria and keep mold from forming. 6-Tert-Butyl-2,4-Xylenol has a record of stopping molds and spores, especially in water-based cleaners or disinfectants. Mold in a bathroom or mildew in a locker room both raise health flags, so formulas using this compound help lower that risk across kitchens and clinics alike.
Chemicals keep our stuff working longer, but the trade-off means thinking about human exposure. Studies collected by regulatory agencies like the EPA and ECHA review chemicals in cleaning and industrial products, including this one, for skin sensitivity or build-up in the environment. Companies adjust levels in their formulas to meet legal safety caps. Product packaging pushes out info, not always at the front in bold print, but the data rolls out for anyone who wants to read the material safety data sheets online. Responsible use ties into safer manufacturing—avoiding overuse keeps air and water clearer, and leaves less mess for drinkable water sources downstream.
Getting caught in the cycle of making plastics tougher and cleaning agents stronger, companies start reaching for whatever slows decay. Relying on 6-Tert-Butyl-2,4-Xylenol sidesteps questions about plastic waste and chemical runoff. Finding options that do the job without sticking in water and soil is the future path. Green chemistry takes this challenge seriously, backing the hunt for antioxidants that break down or return to nature safely. In the meantime, regular folks can ask for transparency—clear labels and data, companies on the hook for responsible disposal, and laws that put people and ecosystems before convenience. Choosing wisely extends past our garage tools or soap bottles, touching everyday health and resource use as well.
People often see chemical names and think of classrooms or high-tech labs, but 6-Tert-Butyl-2,4-Xylenol pops up in coatings, adhesives, and even consumer products. I’ve worked in places that push speed and cost, but conversations around chemical safety don’t happen as much as they should. Overlooking safety can invite problems you never see coming.
I once saw a co-worker skip gloves “just for a minute.” That small moment led to a trip to occupational health with red, itching skin. This chemical can cause skin or eye irritation and, with enough exposure, affect your breathing. Jumping right into work without thinking about protection invites risk, even if nothing seems wrong at first.
Rubber or nitrile gloves shield hands from liquid contact. Not all gloves block chemicals, and it’s easy to reach for whatever’s on hand. Take a moment to check the glove’s material. Eye protection isn’t just for splashes—fumes can irritate eyes. Safety goggles with side shields close off common entry points.
Some labs get stuffy, but this chemical benefits from plenty of ventilation. A fume hood pulls vapors away and keeps the air clear. Not every workplace can afford top-end systems, but even a small fan moving air through a window makes a noticeable difference. Mask use depends on the job. Inhalation of powder or vapor happens in a second, so choose a mask fitted for organic vapors whenever there’s a risk of dust or mist.
Storage often gets overlooked in day-to-day tasks. I’ve opened cabinets and caught a whiff of strong odors because someone capped a bottle loosely. This chemical performs best when kept in a sealed container in a cool, dry spot, away from sunlight. Labeling helps a lot, because clear names beat faded, hand-written abbreviations every time.
Spills can mean panic, but a steady approach reduces chaos. Contain the area with absorbent material. Try not to sweep or vacuum unless the equipment is rated for chemical spills—I’ve seen cheap vacuums send powders flying across a room. Toss out anything that soaks up the chemical in a sealed, labeled bag. Wash every surface with lots of soap and water. People tend to forget about proper disposal, but pouring chemicals down the sink may send them straight to the local water supply.
Every lab should have an eye wash and emergency shower close by. If someone gets exposed, rinsing immediately protects the skin and eyes. For breathing problems, get everyone to fresh air right away. Even after things feel normal, a trip to the doctor offers proof you acted responsibly.
Training isn’t just a box-ticking exercise. Walking through procedures as a team ensures nobody forgets the basics in a crunch. Reading the Safety Data Sheet together at the start of a project creates a habit of awareness. Peer reminders help; a simple “Did you glove up?” can prevent a mistake from becoming an accident.
Building a safety culture starts with sharing stories, not just reciting rules. I’ve learned the hard way that a minute’s precaution always costs less than an hour’s clean-up. Common sense grows when people spend less time fearing chemicals and more time respecting them. Real safety depends less on rules taped to a wall and more on habits practiced every day.
Seeing a chemical name like 6-tert-butyl-2,4-xylenol might remind some of confusing moments in high school chemistry—those days spent trying to memorize the difference between methyl, tert-butyl, and the rest. Instead of letting all that slip away into something mysterious and intimidating, a closer look brings out the sense in each piece of this compound’s name.
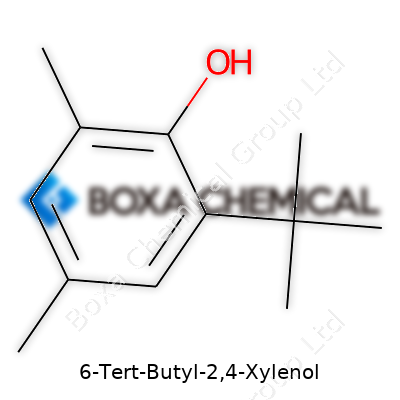
Here’s what’s packed inside: 6-tert-butyl-2,4-xylenol has the chemical formula C12H18O. Nothing fancy—the bones are a benzene ring, which is that common hexagon-shaped backbone in so many molecules. Stick two methyl groups at the 2 and 4 positions on that ring. The “6-tert-butyl” means a tert-butyl group attached at the 6th position, making it a pretty bulky side chain, and “xylenol” tells us there’s also a hydroxyl (–OH) group on the ring. On this molecule, the –OH sits at position 1.
To really picture this molecule, start at the benzene ring. Put a hydroxyl group at position 1. Move to position 2, add a methyl group. Hop over to position 4 and add another methyl group. Get all the way around to position 6 and crowd in a tert-butyl group. This puts the tert-butyl and hydroxyl groups almost across from each other, which gives this compound its distinctive shape and properties.
It looks like this:
If someone draws the structure out, the tert-butyl group stands out—it's bulky and more complex than the usual single methyl. The arrangement crowds the ring, making this molecule less reactive in some directions and more stable than simpler phenols.
Chemical structures like this might not seem electrifying, but they connect to things many people use every day. 6-tert-butyl-2,4-xylenol is known for its role as an antioxidant. This means it keeps other materials from breaking down or changing in the presence of oxygen. It's worked into formulations for lubricants, plastics, and rubbers, keeping these materials usable longer.
This compound shows up in cleaning products and in some industrial processes too. Its antioxidant properties slow down spoilage and help keep products shelf-stable. These little tweaks at the molecular level can lead to longer-lasting car engines, fresher cosmetics, and stronger plastics. It’s hard to appreciate just how much smoother life runs with the help of chemicals like this.
Every useful synthetic compound also comes with concerns. Handling chemicals with bulky groups like tert-butyl demands care. Exposure can raise health questions, especially in workplaces that aren’t focused on protective measures. Too much reliance on antioxidants can cover up deeper production flaws—sometimes, a formulation might benefit more from a cleaner process rather than heavier reliance on additives.
The key lies in responsible use and understanding. Workers need clear safety protocols, including gloves, masks, and good ventilation. Research still pushes for safer, biodegradable alternatives. As attention to chemical exposure increases, expect more pressure toward transparent ingredient lists and a move to antioxidant systems with less environmental baggage.
Chemistry gives materials the staying power we expect. 6-tert-butyl-2,4-xylenol, for all its complicated name and quirky rings, plays one small part in everyday durability. Progress comes as we build both safer practices and better alternatives—making sure the benefits chemicals deliver don’t leave hidden costs for health or the planet.
Anyone who’s ever handled chemicals knows things get real fast if you ignore safety. 6-Tert-Butyl-2,4-Xylenol comes in handy in a bunch of industrial processes, but its value means nothing if poor storage leads to leaks or contamination. Chemical safety doesn’t wait for a bad day—so storing this compound takes focus, common sense, and planning.
Most seasoned techs and lab managers keep 6-Tert-Butyl-2,4-Xylenol in a cool, dry place. Humidity leads to clumping, crusting, or degradation, which ruins purity. Too much heat creates unnecessary pressure or even fumes, which can trigger headaches or worse if you’re on shift with little ventilation. In my own lab days, we kept the container out of direct sunlight, far from the window and heat sources. A climate-controlled storeroom or cabinet knocks out those variables, so it’s not a rolling dice situation every time you reach for the product.
Cheap plastics break down or leach when they meet strong chemicals over time. High-quality, tight-sealing glass or compatible HDPE (high-density polyethylene) containers cost more upfront, but every old-timer in the business will tell you: you either pay for safe storage or you pay for damage control. A well-sealed lid stops air and moisture, keeping xylenol stable and holding its quality for the long haul. Always label these containers—never trust a guess in a storeroom with five similar-looking powders sitting side by side.
Mix-ups between chemicals end in wafting fumes, wasted product, or worse—accidental reactions. Never stack or store 6-Tert-Butyl-2,4-Xylenol next to strong acids, alkalis, or fuels. Segregation matters more than a pretty shelf; it’s about protecting handlers and shipments from a small error turning into an incident. Designated shelves or lockers for different chemical types help young techs stay sharp, and they help old hands keep their routine safe.
Bulk storage often looks cheaper but comes loaded with risks for leaks or accidental overdosing. Decant only as much as your team can safely handle in a reasonable timeframe. Multiple small containers beat one large drum. I’ve seen accidental spills with multi-liter vats that would have been small blots if packed smart. Log how much you have, date every container, and cycle stock so nothing sits forgotten past its shelf life. This practical approach cuts down on surprise messes and keeps inventory manageable.
OSHA and Globally Harmonized System (GHS) guidelines give a reliable foundation, but real skills come from practice and mentorship. Run regular safety sessions on the storage protocol, and ask new staff to shadow a more experienced peer. Safety data sheets should never sit collecting dust—they should hang on the wall where anyone can check them during a crisis. Full PPE is non-negotiable: gloves, goggles, and dust mask, every time. It sounds like overkill until the day a slip happens. Every storage strategy only works if the people follow through, stay alert, and communicate with the next shift.
Each step in safe storage protects workers, equipment, and finished products. Action beats good intentions here: inspect containers, check airflow systems, keep storage logs, and never shortcut the rules. Doing it right saves money by reducing loss and downtime, but it also speaks of respect for the people whose hands mix, pour, measure, and move these chemicals every day. The right approach to storage means the chemical does its job and nothing more—no surprises, just reliability you can trust.
6-Tert-Butyl-2,4-Xylenol might sound like insider chemistry, but it ends up in a lot of industrial products—plastics, rubbers, lubricants, adhesives. I come from a family where my father spent decades on the shop floor, and I remember how mysterious some of the workplace labels seemed to the untrained eye. The glossy data sheets glossed over how a chemical could affect the person standing right there handling it day after day.
This compound, often used as an antioxidant, lands on government hazard lists for a reason. Exposure can irritate the skin and eyes. If you touch it—especially as a fine powder or spray—it can cause redness and even mild burns. I talked to a few folks who run maintenance in chemical plants; one told me he saw a coworker get splashed just once on the forearm and struggle with irritation for hours. Sometimes, itchy or red skin becomes a part of life on certain production lines, and people just call it “the price of the job.” That shouldn’t be the norm.
Vapors from 6-Tert-Butyl-2,4-Xylenol also find their way into the air, especially in poorly ventilated shops. Breathing in those vapors can irritate the throat and lungs. After extended or repeated exposure, some folks report chest tightness and chronic coughing. The National Institutes of Health lists respiratory effects, including aggravated asthma and bronchitis, as possible outcomes for workers exposed over long shifts.
The long-term picture gets murkier. Scientific studies on animals point to concerns about how this compound could affect the liver and kidneys after chronic exposure. No one wants to come home after forty years in a shop and find out their retirement is off track because of silent health damage. Having met retired workers who carry the effects of chemical exposure with them, I often wonder whether their employers flagged every risk on those safety sheets.
For most people, the route of exposure comes through the skin or airways. Regular hand washing and following Occupational Safety and Health Administration (OSHA) guidelines can help, but not everyone receives the right safety training, especially contractors or temporary staff. Personal protective equipment, like gloves and goggles, often gets set aside once it feels inconvenient, even when the risks stick around. Folks need gear that fits and actually makes their work easier, not clumsier. Safety culture only works if every worker can see themselves in it.
Employers need clear labeling on all containers and up-to-date safety data sheets in plain language. In my experience, you can’t count on people memorizing chemical names, so bold hazard symbols and routine refresher safety classes help keep everyone aware. Local exhaust ventilation systems cut down inhalation risk, and some workplaces I visited have started using real-time air quality monitors for peace of mind.
Substituting less hazardous chemicals, where possible, lightens the health burden. Research into safer additives pays off not just for workers but also for managers keeping an eye on insurance costs and absenteeism. It’s always good practice for anyone dealing with 6-Tert-Butyl-2,4-Xylenol to check in with occupational health specialists and call out any new health concerns early on.
If we ignore these risks, history repeats itself. Listening to workers, investing in better ventilation, and prioritizing health over speed lets people do their jobs without sacrificing their future well-being.
| Names | |
| Preferred IUPAC name | 2,4-Dimethyl-6-(propan-2-yl)phenol |
| Other names |
6-tert-Butyl-2,4-xylenol 2,4-Dimethyl-6-tert-butylphenol Topanol A Antioxidant 2246 2,4-Xylenol, 6-tert-butyl- 6-(1,1-Dimethylethyl)-2,4-dimethylphenol |
| Pronunciation | /ˈsɪks tɜːrt ˈbɜːtɪl tuː fɔːr zaɪˈliːnɒl/ |
| Identifiers | |
| CAS Number | 26741-53-7 |
| Beilstein Reference | 1109560 |
| ChEBI | CHEBI:34445 |
| ChEMBL | CHEMBL49094 |
| ChemSpider | 12090 |
| DrugBank | DB14093 |
| ECHA InfoCard | 100.044.911 |
| EC Number | 215-661-1 |
| Gmelin Reference | 80998 |
| KEGG | C19322 |
| MeSH | D014981 |
| PubChem CID | 70289 |
| RTECS number | ZE2625000 |
| UNII | 2B084TKC5S |
| UN number | 3077 |
| CompTox Dashboard (EPA) | `DTXSID3059475` |
| Properties | |
| Chemical formula | C12H18O |
| Molar mass | 206.32 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Phenolic |
| Density | 1.05 g/cm3 |
| Solubility in water | insoluble |
| log P | 3.7 |
| Vapor pressure | 0.000087 mmHg at 25°C |
| Acidity (pKa) | 11.57 |
| Basicity (pKb) | 10.36 |
| Magnetic susceptibility (χ) | −74.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5340 |
| Viscosity | 176 mPa.s (25°C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 318.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -416.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6364 kJ/mol |
| Pharmacology | |
| ATC code | D02AE02 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H410 |
| Precautionary statements | P261, P264, P270, P273, P301+P312, P330, P501 |
| Flash point | Flash point: 132°C |
| Autoignition temperature | Autoignition temperature: 540°C |
| Lethal dose or concentration | Rat oral LD50 1300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1960 mg/kg |
| NIOSH | BY6100000 |
| PEL (Permissible) | No OSHA PEL established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
2,4-Dimethylphenol Butylated hydroxytoluene (BHT) 2,6-Di-tert-butyl-4-methylphenol 2-tert-Butyl-4-methylphenol 6-tert-Butyl-3-methyl-2,4-xylenol |