The story of 4-tert-butylcatechol tracks back to a period when chemists dug deep into the phenolic family searching for compounds that could stretch the shelf life of materials prone to oxidation. Organic chemists in the early 20th century understood that aromatic rings, decked out with hydroxyl choices, placed antioxidants on solid ground. As industrial chemistry grew, new products faced stability challenges, sparking demand for stabilizers that kept molecules intact. Technologists rooted for 4-tert-butylcatechol, since its tert-butyl group shielded the molecule, allowing producers to use it in resins and rubbers with less risk of breakdown. Today’s widespread adoption leans heavily on discoveries born from those first experiments in oxidative stability.
4-Tert-butylcatechol traces back to the phenolic family. It shows up as a crystalline solid or sometimes melts into a faintly yellow liquid as temperatures climb. Producers choose this antioxidant for its knack at halting oxidation in monomers, especially during transport and storage. The value here leans on reliable performance. Industrial labs, ink manufacturers, and polymer chemists all look to 4-tert-butylcatechol when polymers threaten to thicken, clump, or lose clarity. Available commercially in various grades, some batches come ultra-pure for specific research or high-demand processes, while others serve more utilitarian stabilizing purposes. Production scale now stretches worldwide, with large volumes transported under controlled conditions.
What tells you most about 4-tert-butylcatechol is a blend of strong phenolic odor, white to pale-yellow color, and significant resistance to high heat. With a melting point sitting around 52–57°C and boiling sneaking past 280°C, this compound settles in as a reliable solid at room temperature, resisting volatile loss through evaporation in normal factory environments. Catechols draw attention for their two hydroxyl groups at positions 1 and 2, but the bulging tert-butyl group on the para spot gives this molecule loftier solubility in organic solvents while making it less likely to succumb to oxidative stress. Dissolving best in alcohols, ethers, and hydrocarbons, it stays nearly invisible in water solutions. Its chemical backbone appeals to anyone looking for lasting antioxidant protection under harsh conditions, where ordinary phenolics might cave to heat or oxygen.
Manufacturers provide 4-tert-butylcatechol in several purities, usually starting at 99%. Technical sheets mark out specifics—CAS number 98-29-3, molecular formula C10H14O2, and a molecular weight of 166.22 g/mol. Visual checks focus on color and residue, and most labels call out storage in dry, cool, and vented setups. Safety data attaches tightly to every shipment, reflecting both the handling guidelines and regulatory particulars. Quality-control checks hold high priority, chasing contaminants like heavy metals and identifying possible by-products. Quality assurance teams check each drum, bottle, or can, confirming consistency in melting point, color, ash content, and volatility, backed up with batch tracking for transparency.
Synthesis of 4-tert-butylcatechol keeps things rooted in time-tested organic pathways. Chemists start with catechol or related aromatic compounds and introduce the bulky tert-butyl group through Friedel-Crafts alkylation. Using tert-butyl chloride and a Lewis acid such as aluminum chloride as catalyst, this reaction tends to favor the para position thanks to the steric hindrance built into the molecule. Once the alkyl group finds its spot, the process moves to purification, usually through crystallization or distillation, to separate unreacted materials. Larger-scale plants push the process toward greener solvents and efficiency, reducing waste and improving yield.
4-Tert-butylcatechol’s chemical heart beats with reactivity at both the aromatic ring and the phenolic oxygen atoms. Characteristic phenol reactions—like oxidation to quinones—open doors for researchers looking to tailor surface coatings or fine-tune color properties. Reactions with bases give rise to water-soluble salts, which sometimes expand its utility in water-based polymerizations or stabilization efforts. The molecule handles mild nitration, sulfonation, or etherification, letting chemists create derivatives that branch into more specialized antioxidant roles. Reactivity toward electrophiles and radicals remains at center stage, especially as industries seek long-term stability from stubborn antioxidants.
Chemists and suppliers might toss around several handles for 4-tert-butylcatechol. You might know it as TBC, 4-TBC, or even 4-tert-butyl-1,2-benzenediol. Some markets call it tert-butylpyrocatechol or para-tert-butylcatechol. In catalogues, it often pops up with its CAS number to sidestep confusion. Major chemical suppliers tweak names based on grade or intended application, so it’s worth double-checking specifications before pulling the trigger on a purchase.
Handling 4-tert-butylcatechol safely means staying alert to its moderate acute toxicity and potential for skin or eye irritation. Industry experience points to the habit of working with gloves, goggles, and proper ventilation, especially during transfer or weighing. Dust or vapor inhalation does warrant caution, and even low-temperature processing needs local exhaust ventilation. Emergency procedures get written into every facility’s playbook, focusing on quick spill cleanup and proper waste management. Regulatory standards set by REACH, OSHA, and similar bodies lay the legal groundwork, and every batch comes with updated safety data sheets. Training sharpens focus around exposure limits and storage protocols—this keeps health, safety, and the environment better protected.
The most visible use of 4-tert-butylcatechol falls inside the world of industrial polymerization, particularly as a polymerization inhibitor for styrene, butadiene, and other unsaturated monomers. Without antioxidants, these chemicals would polymerize inside storage tanks or transit lines, leading to costly blockages and potential safety hazards. Paint and ink makers praise its stabilizing power, which keeps resins open to curing when needed, not before. Rubber manufacturers add it to latex or finished products, cutting down on early aging and maintaining performance over time. Beyond bulk chemical industries, researchers find space for it in specialty antioxidants, corrosion inhibitors, agrochemicals, dyes, and even selected pharmaceuticals where stability under oxygen-rich environments becomes a challenge.
Research efforts dive into optimizing both production and application. Green chemistry searches for new catalyst systems or cleaner alkylation methods to reduce waste. Academic labs dig into mechanisms—how radicals interact with the catechol core, how substitution patterns tweak antioxidant strength, and how molecular tweaks could create longer-lasting stabilizers. Development teams in coatings or resins push boundaries, loading up modern materials with small amounts of 4-tert-butylcatechol and measuring anything from aging rates to color fastness in aggressive test conditions. R&D stretches into pharmaceutical packaging, food-contact polymers, and electronic materials, hunting for blends that lock in performance over months—or even years—of storage.
4-Tert-butylcatechol does not brush off human health questions easily. Acute exposure in animals suggests moderate toxicity; at high enough doses, effects turn up in liver, kidney, and blood chemistry. Inhalation studies point to lung and upper airway irritation, largely from vapor or fine dust during handling. Skin contact rarely causes severe problems, but chronic exposure drives researchers to double down on safe-handling habits. Cancer and mutagenicity data remain limited, so best practice always leans toward careful control, workplace monitoring, and ongoing review of animal and cellular studies as new data enters the field. Environmental scientists also watch for persistence and aquatic effects, nudging regulatory agencies to call for robust risk assessments.
Next-generation polymer science won’t ease up on demands for stability, so 4-tert-butylcatechol looks well-positioned to hold its place in the toolkit. Sustainable chemistry movements press for greener syntheses, with waste minimization and improved recyclability leading improvement. Automation and real-time process monitoring may trim unwanted exposure and improve yield. There’s buzz in the bioplastics sector—engineers on several continents keep one eye on how antioxidants like 4-tert-butylcatechol interact with new starch- or polylactic-based polymers. Advances in nanotechnology might ignite new uses where molecular-level control over oxidation opens opportunities in electronics or coatings that flex and recover. Research momentum aims for safer, targeted antioxidants, but field experience keeps 4-tert-butylcatechol close at hand for those who need an answer today.
Walk through any plastics factory, paint shop, or rubber processing plant, and stacks of raw materials line the space. Some of them have a shelf life that's measured in weeks, not years. Things can go wrong if chemicals start reacting before companies even put them to work. 4-Tert-Butylcatechol (TBC) steps in as a practical solution that anyone on the factory floor can appreciate. This compound manages to keep monomers and other reactive chemicals from gelling up or spoiling before they're wanted. Think of it as a security guard for ingredients that show up early to the party and can’t seem to mind their manners.
Too much oxygen ruins a lot of things. TBC acts as a stabilizer and antioxidant to keep things from turning rancid or polymerizing when they're not supposed to. In the case of styrene and butadiene, TBC keeps production safe by suppressing premature polymerization—which can shut down entire lines and force dangerous cleanups. The compound’s performance isn’t theory; the U.S. Food and Drug Administration approves limited use in food packaging, recognizing its reliability and minimal long-term risks when handled with respect.
Factories save money and improve safety with every batch that doesn’t self-polymerize on a railcar or storage tank. Shipping styrene across states or countries without stabilizer would be reckless. TBC helps companies prevent accidental fires or loss of control, since runaway polymerization releases heat and fumes that no worker wants to face. It’s the hidden defender in the system—a small investment that keeps things steady.
Researchers use TBC to preserve sensitive chemicals during storage and transport. Paint manufacturers trust this chemical to keep resins and adhesives stable, avoiding expired inventory or failed projects. In the rubber business, a breakdown in storage costs real money, both in material loss and missed deadlines. A friend who works in industrial supply tells me how downstream customers either win or lose based on these small chemistry decisions. Getting the additives right means fewer product recalls and more consistent quality.
No chemical comes without risks. Mishandling of TBC, like letting it contaminate water or failing to store it correctly, can harm wildlife or workers. Truth is, TBC’s benefits depend on training and robust supply chain management. Industry watchdogs have flagged overuse and contamination accidents in the past. Investing in worker education, spill controls, and clear labeling pays off, not just for compliance but also for jobsite morale and trust between suppliers and customers.
Globally, demand for reliable stabilizers grows with consumer expectations. Regulations focus on both product safety and environmental impact, putting some pressure on manufacturers to use best practices. Sharing data between producers and users of TBC, supporting research on safer disposal or greener alternatives, and keeping workers informed can keep technology out of the headlines for the wrong reasons. In the end, knowing where your materials come from and how to handle them makes all the difference between mishap and mastery in modern production.
4-Tert-Butylcatechol turns up as a stabilizer in the chemical industry, especially with monomers or other reactive compounds. It slows down unwanted polymerization, and its value shows up in keeping plants running safely. But any chemist who’s opened a container of this stuff knows: respect goes a long way. Skin exposure, inhalation, or a splash to the eyes can cause much bigger headaches than a ruined batch.
Getting too casual around chemicals like this one? That’s where most accidents start. Good lab coats don’t just stop splashes; they discourage quick, lazy shortcuts. Gloves built for organic solvent resistance keep 4-Tert-Butylcatechol off your skin; nitrile tends to work reliably for short tasks, but thicker neoprene or butyl types provide stronger barrier if handling large amounts or spills. Eye protection counts as more than just goggles—face shields protect against sudden splashing, and don’t forget fit. Many real-world incidents trace back to loose side seals.
Volatile chemicals and strong odors come with routine lab work. Even if this antioxidant doesn’t take over the room, its vapors irritate your airways faster than you’d expect. Proper ventilation, through local exhaust hoods or well-maintained general systems, chips away at both acute exposures and the slow buildup nobody notices until a headache flares. Respirators remain a must for anyone cleaning major spills, swapping filters as required by safety data sheets, not seniority or guesswork.
Don’t keep 4-Tert-Butylcatechol near direct sunlight or heat. Warmth shortens its shelf life and pushes those vapors into the workspace. Containers need tight sealing—not so much for ‘purity’ as for people’s health and equipment longevity. Label everything with big, bold names and hazard symbols. I’ve seen more than one accident where a colleague grabbed the wrong jar because someone thought a post-it note would do the trick.
No one likes to talk about the worst day in the lab, but spills happen. Absorb small quantities with sand or commercial spill pads made for organic chemicals. Waste goes in marked, closed containers for hazardous materials, never into sinks or general waste bins. In my early years, I saw a tech dump a small amount, thinking “it’s just antioxidant.” That set off an odor that sent everyone outside and halted work for a whole afternoon.
Quick response saves eyesight and skin. Splash? Eyes go right to the eyewash for at least 15 minutes. Contact with your hands or face calls for an immediate wash with soap and running water. Never “wait and see”—I’ve seen minor discomfort turn into real injury by ignoring early signs.
Reading a material safety data sheet once doesn’t cut it. Review procedures until they become habit, not just for yourself but for those who work beside you. Ask questions—there’s no shame in wanting a refresher before pipetting something new. Sometimes staff get too comfortable because injuries remain rare. That attitude can shift in a second, and staying prepared keeps those moments from turning tragic.
Anyone who has worked in a lab, factory, or warehouse learns respect for chemicals early on. Every substance shows its quirks. 4-Tert-Butylcatechol, or TBC for short, carries a few habits you don’t forget. This compound preserves many products folks use daily—from plastics and rubbers to fuels—by slowing down unwanted reactions. But left with lousy storage, TBC becomes more trouble than help. Overlooking the details with this chemical can cause headaches for workers and headaches for those living nearby. I’ve seen a storage room become unusable for weeks as crews fixed a leak that bad planning could have avoided. Mistakes pile up fast with strong substances like this.
TBC only looks calm on paper. It comes as brown crystals or a thick liquid, and just touching air or light stirs up slow changes. Raw sunlight and warmth speed its breakdown, which sets off peroxides—those nasty, unpredictable chemicals best kept far away. Ignoring those warning signs is risky. Maintaining stability tops the list for reliability and safety. This isn’t just about following the rulebook—it keeps everyone who handles it out of trouble’s way.
Shoving a drum of TBC behind a door and calling it “safe” doesn’t hold up. Workers store this product in tight, well-marked containers built for handling chemicals—not any old metal pail. Those containers keep oxygen and moisture out, as even a little exposure sparks slow decay. I’ve witnessed places where even a week in a leaky plastic jug left TBC discolored and clumpy. Proper storage always uses dark glass or drum containers and strong seals. It saves money, too, since spoiled TBC wastes both raw materials and work hours. Smart storage practices protect both the chemical’s quality and business’s bottom line.
Temperature stays important. TBC needs a cool, steady spot—nothing above room temperature. Crowded storerooms with unreliable AC push up the risk for breakdowns and produce extra fumes. If temperatures creep up or drop below freezing, the compound starts turning messy. Hot weather can trigger pressure build-up inside containers; cold weather leads to hardening and hard-to-handle residues. Good ventilation in the storage area keeps fumes from building up and protects the people working nearby. Over my years in labs and industrial plants, a steady storage climate made the difference between safe use and costly mistakes.
Even with the best plans, spills happen. Every storage site needs basic safety gear on standby—eye wash, absorbent material, gloves, protective goggles. Clear emergency procedures keep incidents short and prevent long clean-up battles. Regular checks for leaks or corroded containers catch small problems before they become disasters. The real world rewards vigilance—routine checks and proper signage beat good luck every single time.
Education always pays off. New staff (and veterans, too) need reminders on what TBC does to skin and lungs. Those warning labels and handling sheets may collect dust, but ignoring them invites trouble. Sharing lessons from past incidents helps shake out bad habits before they cause harm. I’ve seen teams become much more careful after even small incidents, as the costs—in lost time, damaged equipment, or health scares—become real to everyone involved.
In the end, real-world storage means respect. Professionals who handle TBC know lazy storage invites expensive, dangerous problems. Good planning earned through practical experience and paying attention to detail keeps everyone safe and operations smooth.
Factories have leaned on 4-Tert-Butylcatechol—often called TBC—for decades. Chemists use TBC as a stabilizer to keep materials from going bad before they reach the customer. Industries dealing with rubber, plastics, and even fuel find its antioxidant properties helpful. TBC can stop chemical reactions that might spoil a batch of product—or even start a dangerous fire. Having worked around industrial labs, I found out fast how TBC keeps things safer on the floor. The trouble starts when we look at how it affects people and the planet.
Anyone handling chemicals like TBC needs more than just a pair of gloves. Close encounters with the powdered form or vapor can irritate skin, eyes, and the delicate lining inside the nose. Breathing it in for long periods could lead to throat pain, coughing, and in some rare cases, trouble breathing. Splashes on the skin might burn or cause rashes. The European Chemicals Agency listed TBC as hazardous, citing its effects on organs if folks get exposed over months or years.
A study from 2018 found lab workers who handled TBC without using masks or proper fume hoods reported frequent headaches and dry skin. To put it plainly, chemicals like this don’t belong on exposed hands or near bare eyes. Some trial data in animals surfaced concerns about the liver and kidneys after repeated, heavy exposures. Even casual contact isn’t risk-free without good habits and personal protective equipment.
Dumping TBC down drains or out with regular waste isn't just careless—it can be damaging. Once it leaks into the soil, it sticks around longer than many common chemicals. Water plants and fish exposed to trace amounts end up absorbing it; this can weaken their immune systems or even mess with their ability to reproduce. In rivers and lakes, small crustaceans proved more sensitive than fish or plants, sometimes dying at concentrations that might not look scary on a label.
Waste authorities in the US and Europe rate TBC as hazardous. If a spill happens, cleanup crews need to use specific methods to prevent water contamination. I’ve seen the difference between a well-managed chemical store and a leaky, untrained operation. In the messy ones, TBC pooled around drains and turned up in soil tests months later.
The answer isn’t simply banning TBC outright, since so many industries rely on it for safety and quality. Instead, manufacturers need to make sure training covers every step—storage, spills, even disposal. Engineering controls, like local exhaust systems, make a difference. Using alternatives where possible isn’t always simple, but labs and factories should keep researching less hazardous stabilizers.
Shrinking the risk comes down to common sense and clear rules. Regulators push for improved packaging to reduce leaks, better labeling to warn users, and limits on disposal methods. From experience, I can say PPE isn’t optional, especially where powders and vapors can drift. On the environmental side, keeping TBC away from the water system stands as the most reliable protection for wildlife and people living nearby.
With up-to-date safety data sheets, honest hazard communication, and strong oversight, the risks linked to TBC don’t have to turn into harm. Still, the story of TBC reminds us that what happens in the lab or factory never stays there—it travels home, downstream, and out into the world.
4-Tert-Butylcatechol (TBC) has a place on nearly every industrial chemist’s list of stabilizers and antioxidants. Over the years, I’ve worked in research labs and have seen firsthand how folks overlook the shelf life of specialty chemicals. The label might give an expiration date, but few pause to wonder what’s behind that number. In truth, safe storage and use come down to more than an arbitrary date stamped on a drum.
Most suppliers put TBC’s shelf life at around one to two years under typical conditions. That’s not just for paperwork—physical stability drops off fast in the wrong setting. Moisture and heat chip away at its potency, and as this compound degrades, it loses its ability to resist oxidation. It’s not just a “chemical” sitting there. It’s a frontline safeguard in styrene, butadiene, and other monomer storage. Fresh TBC can block runaway reactions. Old stock, weakened by time or bad storage, might step aside just when you need it most.
From a safety angle, it’s more than product loss at stake. Outdated TBC means less protection against polymerization, raising risks during shipping and warehousing. That risk isn’t theoretical. Incidents in the past show that unstable monomers can ignite or polymerize—leading to financial loss, injuries, or worse. So, keeping TBC within its prime shelf life isn’t just best practice. It’s good stewardship and basic risk management.
TBC asks for simple but strict storage—keep it cool, dry, and in sealed containers. Exposing it to air speeds up oxidation. Any time you open a drum or bottle, the clock ticks faster. My coworkers and I always watched for color changes or odd smells. That can flag contamination or breakdown. Rigid labeling and a good tracking system helped avoid accidents and waste.
Relying on “just-in-time” procurement reduces the temptation to stockpile this material. Buying only what you can use in the short term keeps TBC fresher. Old habits die hard, though. People love bargains and bulk orders. Still, I’ve seen more headaches from expired or off-spec chemicals than from a few extra purchase orders spread over a year.
Setting up an audit trail pays off. I remember getting a shipment of TBC that looked okay but tested low for active ingredient. Turned out, it had sat in a regional warehouse—unrefrigerated—for months. These events aren’t rare, especially where hot summers and humidity are facts of life. Most global suppliers now offer Certificates of Analysis with each batch. A trained staff reads and acts on these documents, not just files them away. That’s one thing good manufacturers and responsible downstream users share.
Regulators and industry groups like the American Chemical Society call for routine inventory checks and regular chemical rotations on the shelf. With TBC, a best-used-by approach isn’t just red tape. People who ignore it find out the hard way. Efficient storage means fewer expensive mistakes, and living by those dates supports safer, more dependable plant operations.
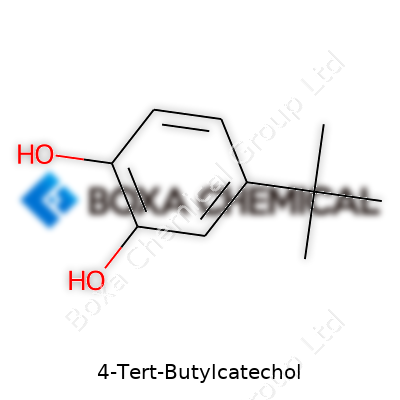
| Names | |
| Preferred IUPAC name | 3-tert-butylbenzene-1,2-diol |
| Pronunciation | /ˈtɜːrt-ˈbɜːtɪl-kəˈtiːkɒl/ |
| Identifiers | |
| CAS Number | 98-29-3 |
| Beilstein Reference | 1209373 |
| ChEBI | CHEBI:34718 |
| ChEMBL | CHEMBL270876 |
| ChemSpider | 12037 |
| DrugBank | DB14015 |
| ECHA InfoCard | 100.017.736 |
| EC Number | 205-426-2 |
| Gmelin Reference | 72478 |
| KEGG | C06505 |
| MeSH | D017619 |
| PubChem CID | 8277 |
| RTECS number | UX9625000 |
| UNII | WAE8A9J6ZW |
| UN number | UN2657 |
| Properties | |
| Chemical formula | C10H14O2 |
| Molar mass | 166.22 g/mol |
| Appearance | Light yellow to amber liquid |
| Odor | Phenolic odor |
| Density | 0.969 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 1.8 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | 11.5 |
| Basicity (pKb) | 11.86 |
| Magnetic susceptibility (χ) | -63.0e-6 cm³/mol |
| Refractive index (nD) | 1.541 |
| Viscosity | 8.6 cP (20°C) |
| Dipole moment | 2.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 327.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -362.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5035.7 kJ/mol |
| Pharmacology | |
| ATC code | D27 |
| Hazards | |
| Main hazards | Harmful if swallowed, toxic in contact with skin, causes skin irritation, causes serious eye irritation, may cause an allergic skin reaction, toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H312, H315, H319, H332, H373 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P308+P311 |
| NFPA 704 (fire diamond) | 3-2-2-W |
| Flash point | > 124 °C (255 °F; 397 K) |
| Autoignition temperature | 530 °C |
| Explosive limits | Explosive limits: 1.1–6% |
| Lethal dose or concentration | LD50 oral rat 800 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 375 mg/kg |
| NIOSH | GG4200000 |
| PEL (Permissible) | Permissible Exposure Limit (PEL) for 4-Tert-Butylcatechol: "5 mg/m3 |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 20 ppm |