People started working with catechol derivatives more than a century ago. 4-Methylcatechol came onto the radar as researchers isolated native plant compounds and began to notice the different functions of methyl-substituted catechols. Lab synthesis allowed chemists to tweak these molecules, giving rise to interest in their antioxidant activity. By the 1970s, interest in phenolic compounds surged, not only in organic synthesis but also for their effects on biological systems. Over time, the scientific community mapped out the roles these substances played in industrial and pharmaceutical settings. Looking at decades of published work, 4-Methylcatechol found itself mentioned in patents involving pesticides, antioxidants in polymer processing, and even as an intermediate in dye manufacturing.
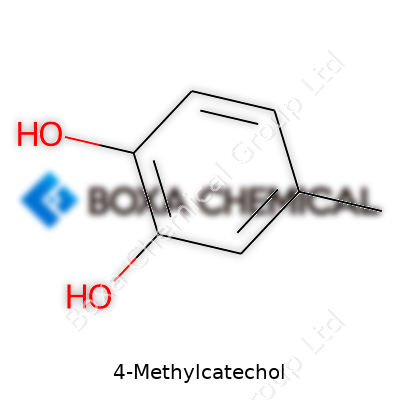
4-Methylcatechol, also known as 3,4-dihydroxytoluene, sits among the methylated catechol family. It’s a solid at room temperature, usually appearing as off-white to beige crystals or powder. Researchers and manufacturing specialists have relied on 4-Methylcatechol as a key intermediate for synthesizing more complex molecules used in fragrances, drugs, and various fine chemicals. Beyond industry, biologists look into its presence and role in living organisms, since it's a metabolite of some natural and synthetic compounds. Its electron-donating properties have attracted attention for experiments involving radical scavenging.
4-Methylcatechol has the molecular formula C7H8O2 and a molar mass of about 124.14 g/mol. It melts at roughly 88°C to 92°C and shows noticeable solubility in water, alcohol, and ether due to its two hydroxyl groups. You won’t catch a particularly strong odor from pure product, unlike some aromatic compounds. Chemically, the molecule resists mild acids but readily undergoes oxidation, which forms colored quinone-type compounds. Color stability becomes a problem under open-air conditions. Proper airtight storage matters because it absorbs moisture and discolors when exposed.
Reliable suppliers deliver 4-Methylcatechol with purity ranging from 98% to 99% for research and manufacturing. Data sheets often mention identifying tests through melting point determination, thin-layer or high-performance liquid chromatography, and spectral fingerprints (NMR, IR, UV-Vis). Physical contaminants such as ash, water, and volatile impurities can compromise product quality if not tightly monitored. Labels in industrial shipments have to show the UN number (for hazardous goods), proper hazard statements, batch number, purity, and date of manufacture. Major jurisdictions like the EU and US require compliance with chemical safety management regulations, so every bottle or drum comes with an updated SDS (Safety Data Sheet).
The main route for making 4-Methylcatechol at scale starts from toluene. First, selective nitration provides 4-nitrotoluene. Chemists then reduce the nitro group to an amine, which undergoes diazotization. Coupling this intermediate with water under mild acid gives 4-methylphenol, and from there, forced ortho-hydroxylation—often using hydrogen peroxide in the presence of iron or copper catalysts—yields 4-Methylcatechol. Small lab-scale runs may take shortcuts, but large-scale plants follow robust multi-step syntheses to maximize yield and purity. Waste streams from the process contain organic solvents and acids, which operators treat and monitor to keep environmental impact in check.
In the lab, 4-Methylcatechol reacts predictably at both its phenolic sites and the methyl group. Under mild oxidation, it turns into a methylated benzoquinone. Electrophilic substitution fits well at the aromatic ring, especially at the 5-position, letting chemists install sulfonic or nitro groups. Alkylation turns the active phenols into ethers, making them less prone to oxidation. In biological settings, enzyme-catalyzed methylation, glucuronidation, or conjugation with sulfate also transform 4-Methylcatechol. These pathways help scientists track its metabolic fate in plants, animals, and engineered microbes. Chemical modifications continue to attract drug discovery teams looking to create analogues with better activity profiles or reduced toxicity.
Beyond 4-Methylcatechol, you’ll see this molecule labeled as 3,4-dihydroxytoluene, 4-methyl-1,2-benzenediol, or p-methylcatechol in scientific catalogs and technical literature. Trade names or in-house codes in a chemical company’s portfolio can cause confusion for new technicians, so clarity in paperwork matters. PubChem and ChemSpider assign it unique identifiers, and regulatory frameworks tie hazard information to these synonyms to avoid mislabeling incidents.
Anyone handling 4-Methylcatechol needs to pay attention to chemical hygiene. Although its acute toxicity sits low, repeated exposure can irritate skin, eyes, and mucous membranes. Inhalation of dust or vapors causes respiratory discomfort, and spills in the workspace stain surfaces and hands. Companies require workers to use gloves, lab coats, goggles, and local exhaust ventilation. Emergency procedures call for eye wash and shower stations nearby. Waste handling teams neutralize residues before consigning them for disposal, and they monitor air quality for traces of volatile phenolic compounds. Compliance with REACH in the EU and EPA regulations in the US has tightened record-keeping and workplace monitoring.
4-Methylcatechol finds employment in multiple segments. Chemical plants use it as a raw material for antioxidants during rubber and polymer stabilization. Pharmaceutical developers test its derivatives for anti-inflammatory, anti-platelet, and possible neuroprotective properties. In the fragrances sector, minor tweaks to its structure yield aroma chemicals for perfumes and flavorings. Some dye manufacturers exploit its electron-rich aromatic ring to build advanced colorants. On the research level, biochemists include it in studies on oxidative stress due to its free radical-scavenging nature. Agrochemical explorers have looked at certain methylcatechols for their pesticidal or plant growth-regulating properties.
For people working in R&D, 4-Methylcatechol offers a balance of chemical versatility and biological potential. Scientists continue to scan it for pharmacological effects, hoping to discover new anti-cancer or anti-microbial activity. Analytical chemists use it as a model substrate for studying phenol oxidases and peroxidases, enzymes that matter in medical diagnostics and food spoilage. Polymer scientists explore ways to harness its antioxidant function by embedding it into plastics, extending product shelf life. Research teams often collaborate across academia and industry to create new derivatives or find eco-friendlier manufacturing routes, aiming for better atom economy and fewer environmental side effects.
Toxicologists track both acute and chronic effects. In rodents, researchers notice mild toxicity only at high doses. Studies point to mild elevation of liver enzymes and some evidence of DNA damage at massive overdoses, data that regulators consider when setting workplace exposure limits. Scientists studying environmental impact report that high concentrations harm fish and aquatic bugs, so wastewater controls become non-negotiable. Metabolic studies in mammals show that after ingestion, 4-Methylcatechol gets rapidly metabolized, conjugated, and eliminated in urine, which helps reduce risk under controlled use but doesn't mean hands-off handling. Some groups test analogues for improved safety profiles before proposing any new industrial applications.
Industry eyes 4-Methylcatechol for building blocks in safer pharmaceuticals and high-performing antioxidant additives. Enzyme engineering teams look for biocatalytic routes that cut down on chemical waste. Green chemistry principles could spark new syntheses, driving down costs and raising the bar for sustainability. As more researchers investigate the health effects and environmental behavior of methylated catechols, regulations will likely become tighter on emissions and worker exposure. Specialists believe that, with deeper insight, safer processing, and targeted modifications, this compound will continue to matter for specialty chemicals and biomedical innovation alike. Cross-sector partnerships, better real-time monitoring, and investment in R&D could unlock new uses—pairing economic growth with greater confidence in human and environmental safety.
4-Methylcatechol hits the market as a chemical with a few specialized, yet meaningful, roles. Some people know it as a synthetic molecule, but it also shows up in certain plants and foods—like coffee and onions—on a much smaller scale. In my work with research labs, this name pops up most in technical documents focused on pharmacology and biochemistry.
The thing about 4-Methylcatechol: it’s a tool for scientists who dig into the way nerves grow and survive. Those in neurobiology circles have put this compound to work for decades because it mimics a natural brain chemical—catechol. In animal studies, researchers test how 4-Methylcatechol ramps up nerve growth factor (NGF), a protein essential for keeping certain neurons healthy. This type of lab work points to ways of slowing the development of diseases like Alzheimer’s, where nerves waste away well before their time.
Most regular folks won’t bump into bottles of 4-Methylcatechol at a pharmacy, and the compound isn’t on drug store shelves. It mostly helps as a research tool for testing how the body responds to stress at the molecular level. Researchers count on it to stir up findings that might become tomorrow’s treatments for nerve damage or degenerative diseases.
Out in industry, 4-Methylcatechol gets more low-key attention. Chemical manufacturers use it in the early stages of making dyes and antioxidants. A chemist working at a dye plant may reach for this material when crafting products that end up coloring fibers or plastics. The antioxidant side matters, too, since the chemical structure allows it to slow down reactions that damage materials over time.
Lab analysts often rely on 4-Methylcatechol as a reference or standard for measuring small amounts of similar compounds in food or water. In food testing, the compound’s presence points to certain reactions or changes in crops—with coffee, for example, slight variations in the roasting process may change how much is present in the final bean. Analytical labs watch for these signals to make sure quality stays where it should.
Most people might never notice the name 4-Methylcatechol in daily life, but a closer look into its uses reveals its ripple effects. A university lab might use this molecule to sharpen knowledge about how brain damage can be stopped or reversed. At the same time, quality labs depend on it for accuracy in food safety or environmental monitoring.
Handling this chemical takes care. Strict limits govern how much can end up in foods because — like many lab-made phenols — it brings risks if it builds up in the body. Government regulations and watchdog organizations keep a close eye for consumer safety. For every promising use in research, there’s a need for clearer long-term studies to back up findings before anything hits the market for medical use.
As researchers dig deeper, it’s clear that 4-Methylcatechol keeps helping scientists unlock secrets about the body’s wiring. Of course, moving from animal studies to treatments that truly help people means answering some tough questions about side effects and long-term impacts. So far, most work stays in the lab. But every new experiment adds to the story, inching closer to breakthroughs in both medicine and industry. Those working with this chemical need — and deserve — the best safety protocols and honest oversight to move the science forward the right way.
4-Methylcatechol shows up in labs and chemical plants as a crystalline compound most folks in organic chemistry circles know. If you've ever worked in a lab, the aroma that hits your nose when handling phenolic chemicals sticks with you. Sometimes that smell means trouble, so folks wearing gloves and goggles already guess caution is the rule.
I've handled all sorts of reagents during college research and in industry, and the safest habit is treating every unfamiliar chemical as risky. For 4-Methylcatechol, safety data sheets tell us it can affect both skin and respiratory systems. My own skin’s gotten burned once by splashed organic material—I didn’t enjoy learning that lesson, and there’s no good reason someone else should have to learn it the hard way. Handling 4-Methylcatechol with exposed skin or breathing in dust means you risk irritation or worse.
According to published toxicological studies, this compound can provoke skin, eye, and lung irritation. Longer exposure, especially if you’re unlucky enough to inhale it, can bring on headaches or trigger allergic responses in some folks. The European Chemicals Agency flags 4-Methylcatechol for its toxicity if swallowed, so it doesn’t belong anywhere near food prep areas. More serious complications come up if exposure lasts for long stretches: reports suggest repeated contact could harm your blood or liver.
From a practical side, the stuff demands gloves and eye protection at a minimum. Fume hoods weren’t just built to make labs look high-tech—turning that fan on keeps invisible dust or fumes out of your lungs. I remember working with phenolic reagents under supervision; we checked our gloves twice before even opening the bottle.
Don’t brush off chemical hygiene. Regular handwashing, proper labeling, and clean benches keep a minor spill from becoming a panic. If you get any 4-Methylcatechol on your hands or in your eyes, running to the eyewash station or using emergency showers isn’t being cautious—it's just standard protocol. I saw a colleague ignore small splashes once, and their afternoon involved a trip to the doctor.
People running student labs know you can stack all the warning stickers in the world on a bottle, but safe handling starts with clear training. New employees or students picking up pipettes for the first time should see how spills get managed and where protective gear sits. Some workplaces slip on refresher courses, and that leads to mistakes. In my experience, the labs where folks routinely run short drills on spills or burns face fewer incidents.
Investing in well-ventilated workspaces helps. Reliable local exhaust and functioning fume hoods make a difference. Facilities should keep less of this chemical on hand unless ongoing work needs it. Some researchers push for less hazardous substitutes, and that’s worth exploring when feasible, especially if similar results can be had with a safer reagent.
Disposing of phenolic wastes responsibly—never down the drain—remains critical. Regulations in most places underline that, but practical supervision enforces it. If your lab stores chemicals like 4-Methylcatechol, regular inventory checks and timely disposal of outdated stock avoids accidents.
Checked sources like the U.S. National Library of Medicine’s TOXNET or the European Chemicals Agency provide clear hazard details and instructions for 4-Methylcatechol. Reviewing this data with each shipment helps prevent errors. Counting on up-to-date information from reliable sources beats guessing or word-of-mouth advice every time. Real safety owes more to habits and teamwork than to any single warning sign or label.
Chemistry in daily life often seems like a distant world, full of jargon and intimidating symbols. 4-Methylcatechol, despite its formal name, plays a basic role in fields like medicine and environmental science. Its chemical formula, C7H8O2, offers more than meets the eye. Each letter and number tells a story about the molecule's formation and use.
C7H8O2 describes a benzene ring carrying two hydroxyl groups and a methyl group. That’s three key changes on a simple carbon ring, dramatically shaping how the compound behaves. The hydroxyl groups make it reactive, prone to forming new bonds. The methyl group, set at the fourth position, subtly changes the molecule’s effects and uses.
As a writer who’s sat through too many lectures, I still remember realizing how much small tweaks in molecules matter. 4-Methylcatechol is a case in point: those two oxygen atoms, separated by a methyl group, open up reactions in biotechnology and industry. Researchers see it as a starting ingredient for antioxidants, medicines, and even dyes. Each compound can help prevent oxidation, a process that makes food go bad or causes cells to age.
These natural derivatives show up in fruits and vegetables. For example, they are related to compounds released when apples or pears turn brown after being cut. That trick nature does with small molecules, turning them into vital signals or protective agents, lies at the core of biochemistry research.
The structure of 4-Methylcatechol guides its safety profile. The presence of those two hydroxyl groups means it can react easily, sometimes forming more dangerous compounds if mishandled. Some studies have found that related catechols might irritate skin or affect air quality during production, so safety precautions always make sense. Reliable sources, like the International Agency for Research on Cancer, keep tracking these exposures to safeguard workers and communities.
It pays to use well-ventilated workspaces and wear gloves or goggles when working with dowel chemicals. Small changes in industrial protocols—like shifting to closed systems—have helped reduce risk. Green chemistry approaches, aiming to create and use less hazardous derivatives, also take the spotlight. By tweaking the structure, researchers develop compounds with the same helpful properties but fewer negative effects.
It’s easy to gloss over a chemical formula as just a puzzle or a random arrangement of symbols. In truth, each formula, like that of 4-Methylcatechol, combines discovery and practicality. Understanding how these molecules fit into our environment leads to safer labs, better medicines, and even improvements in the food on our tables. Each tiny improvement comes from paying attention to details, not just in symbols on a page but in health and daily life.
In any laboratory or facility where chemicals show up on a regular basis, one of the main concerns always circles back to storage. 4-Methylcatechol isn’t an exception. This compound drops into warehouses and research labs with little preamble, looking harmless but packing enough reactivity to cause trouble if left unchecked. In my years running a college teaching lab, I’ve found routines are only as good as the attention people pay to details like storage temperature, container material, and room traffic—especially with sensitive compounds like this one.
4-Methylcatechol breaks down if exposed to air or moisture for long periods. This isn’t a minor problem; instability leads to safety hazards, ruined samples, or invalid research data. Accidental contact with moisture in the air kicks off oxidation, and before long, you’re staring at a ruined batch that might even irritate sensitive skin or damage surfaces. Skipping careful storage basically writes an open invitation for these problems to walk through the door.
Sealed, airtight containers form the first line of defense. Glass containers with screw-top lids lined with PTFE work well. Plastic doesn’t always cut it here since certain plastics can leach or react with small organics after a while. Every batch I’ve ordered or prepared got stored in glass in a clear part of the chemical storage room, away from direct light and out of any humid corner.
It helps to keep the temperature consistent, too. Cool, dry places slow down the usual degradation reactions. Standard lab protocol—keep all air-sensitive chemicals away from heat sources, windows, and the warmth of running equipment. Refrigerators built for chemical storage keep things below room temperature but well above freezing; this reduces reactivity without risking condensation inside the container.
Labeling might sound obvious, but missing dates or hazard tags spell disaster, especially if new staff or students cycle through lab space often. Every container gets a clear label with the arrival date, chemical name, and applicable hazard symbols. Simple habits reduce mix-ups or forgotten chemicals collecting dust until someone finally gets a nasty surprise.
Sensible storage rules stop at nothing short of being strict. Many chemicals, including 4-Methylcatechol, let off fumes that can build up. Good ventilation helps—I’ve always kept similar organic compounds on shelves in ventilated cabinets or fume hoods. Chemical-resistant spill trays underneath catch leaks or tiny spills before they do bigger damage.
Regular checks make the system work for everyone. Scheduling periodic inspections to look for broken seals or suspicious discoloration keeps supplies safe to use. No one likes running out of stock or sending spent chemicals to special disposal because of sloppy care. Teams who take safety seriously cut down on emergencies and keep their labs running smoothly.
Safe, organized storage is more than a rule. It’s about protecting people, budgets, and results. Every time a class or team handles a bottle of 4-Methylcatechol stored properly, it shows foresight and respect for the work. Reliable storage prevents loss and supports everyone—staff, students, and the work they’re proud to produce. For any lab, keeping chemicals like this in check proves that a little planning always beats scrambling to fix preventable mistakes later on.
4-Methylcatechol pops up in labs and certain industrial settings, usually as an intermediate for dyes, pharmaceuticals, and antioxidants. For most people, this name doesn’t ring a bell, but folks working in research or manufacturing run into it more than they’d like. Its structure closely resembles catechol, yet introducing a methyl group changes more than just its name. Sometimes, small tweaks in structure unlock new health risks.
I’ve handled my fair share of chemicals, including catechols. Soon as 4-methylcatechol lands on skin, irritation strikes fast. Redness, itching, and even blistering can flare up in minutes, especially after repeated exposure. Inhaling dust or vapor attacks the eyes and respiratory tract. Breathing gets rougher, coughing follows, eyes water. It feels like you’re standing next to a leaky bottle of ammonia. Even small spills turn into a big problem if folks ignore protective gear.
Here’s the thing: acute discomfort runs its course, but long-term exposure rarely gets enough attention. Based on animal studies and what’s known about related catechols, persistent low-dose exposure stirs up more chronic issues. Inhaling or absorbing catechols can lead to kidney stress, and the liver takes a hit, too. The chemical works its way into the bloodstream, putting extra load on detox pathways. During my grad school years, a safe lab meant keeping direct contact to zero, even with fumes. Good ventilation and swift cleanup routines didn’t just follow regulations—they kept us healthy for the long haul.
This chemical sits in a gray area for cancer risk, but some evidence from related compounds waves a red flag. Catechols participate in oxidation-reduction cycles, kicking up reactive intermediates. These can bind to DNA, raising the possibility of mutation. The International Agency for Research on Cancer keeps an eye on catechols due to their ability to trigger oxidative stress, a sneakier route to chronic disease. More research must nail down the exact risk, but caution wins the day.
It’s not just about direct contact or workplace accidents. Wastewater and improper disposal send 4-methylcatechol downstream, threatening aquatic life. Fish exposed to trace amounts suffer from gill damage and nervous system stress. That same water gets recycled into crops and drinking water for both animals and humans. I grew up in an area where factory runoff left a mark on local ponds—years later, health issues popped up without warning among neighbors who never set foot in those factories. Environmental health stays wrapped up with human health.
Best protection comes from reliable barriers—gloves, goggles, lab coats, and tight containers. Proper ventilation keeps fumes from spreading. Training every new hire in chemical hygiene and fast spill response cuts down incidents. Waste management companies must track and neutralize every liter heading out of industrial sites. Even schools and universities housing small stockpiles need policies matching big industry standards. Speaking up about safety supplies and procedures sometimes saves a lot of grief.
Using 4-methylcatechol brings knowledge, respect, and a willingness to ask tougher questions about long-term risks. New research deserves encouragement, but every workplace or town sharing a zip code with chemical storage ought to treat exposure vigilance as part of daily life. No job or discovery rates higher than health tomorrow or twenty years from now.
| Names | |
| Preferred IUPAC name | 4-methylbenzene-1,2-diol |
| Other names |
4-Methylpyrocatechol 3,4-Dihydroxytoluene Pyrocatechol monomethyl ether |
| Pronunciation | /fɔːr ˌmɛθ.ɪlˈkæt.ɪ.kɒl/ |
| Identifiers | |
| CAS Number | [452-86-8] |
| 3D model (JSmol) | `3D model (JSmol)` string for **4-Methylcatechol**: ``` 14 4-Methylcatechol C1=CC(=C(C=C1O)O)C ``` This is the SMILES string used for generating the 3D model in JSmol. |
| Beilstein Reference | 1361179 |
| ChEBI | CHEBI:34577 |
| ChEMBL | CHEMBL44900 |
| ChemSpider | 7288 |
| DrugBank | DB02957 |
| ECHA InfoCard | 03b0fd5f-9bef-408f-96e4-ae9582d0c335 |
| EC Number | 1.10.3.1 |
| Gmelin Reference | 92105 |
| KEGG | C06505 |
| MeSH | D08.811.034.132.150 |
| PubChem CID | 8691 |
| RTECS number | GG8050000 |
| UNII | L5K7J5GSNQ |
| UN number | 2811 |
| Properties | |
| Chemical formula | C7H8O2 |
| Molar mass | 124.14 g/mol |
| Appearance | White to beige solid |
| Odor | phenolic |
| Density | 1.129 g/cm³ |
| Solubility in water | soluble |
| log P | 0.21 |
| Vapor pressure | 0.0018 mmHg (at 25 °C) |
| Acidity (pKa) | 9.58 |
| Basicity (pKb) | 9.37 |
| Magnetic susceptibility (χ) | -79.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.565 |
| Viscosity | 1.84 mPa·s (20 °C) |
| Dipole moment | 1.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 119.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -101.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3232 kJ/mol |
| Pharmacology | |
| ATC code | N02BX08 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, may cause damage to organs through prolonged or repeated exposure. |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0-NULL |
| Flash point | 79 °C |
| Autoignition temperature | 615 °C |
| Explosive limits | Explosive limits: 1.4–7.0% |
| Lethal dose or concentration | LD50 (oral, rat): 283 mg/kg |
| LD50 (median dose) | 400 mg/kg (Rat, oral) |
| NIOSH | SN 2100000 |
| PEL (Permissible) | PEL (Permissible) for 4-Methylcatechol: Not established |
| REL (Recommended) | 10 mg/L |
| IDLH (Immediate danger) | IDLH: 150 mg/m³ |
| Related compounds | |
| Related compounds |
Catechol 4-tert-Butylcatechol Guaiacol 4-Chlorocatechol 4-Methylguaiacol |