Long before scientists identified the chemical backbone of 4-Ethylguaiacol, people recognized its distinct aroma without naming it. For centuries, winemakers and brewers smelled spicy, smoky undertones in their casks. Only in the twentieth century did advances in analytical chemistry reveal this particular molecule as a key contributor to certain flavors in wine, whisky, and beer. Isolation and synthesis of 4-Ethylguaiacol opened a path for detailed studies. This compound’s early journey ran parallel with the evolution of gas chromatography, which allowed researchers to pinpoint and quantify complex flavor components. Over time, its reputation grew—sometimes as a sought-after flavor, sometimes as a defect. This duality drove interest in understanding its origins and consequences. As microbial control in fermentation improved, so did awareness around managing levels of 4-Ethylguaiacol across the beverage industry.
4-Ethylguaiacol belongs to the class of alkylated phenols. Manufactured either for research or as a flavor additive, it appears in the form of a colorless to pale yellow liquid. Suppliers tailor its purity depending on use, with analytical standards reaching upwards of 99%. In the beverage world, even small concentrations can transform taste or aroma, which makes control and predictability critical. Not only does it occur naturally, but it also gets produced synthetically for consistency and safety. Laboratories source it for calibration, while some food and fragrance companies use tiny doses to tweak sensory profiles. Understanding where it fits leads to wiser use and effective quality control.
4-Ethylguaiacol carries the molecular formula C9H12O2 and weighs in with a molar mass of 152.19 g/mol. At room temperature, it presents as an oily liquid, its boiling point reaching around 247°C. This compound dissolves well in organic solvents like ethanol or ether, but only sparingly in water. What really stands out is its olfactory threshold: even tiny amounts—often below 600 parts per billion—get detected by the human nose. Its aroma swings from spicy clove to smoky bacon depending on concentration and environment. With a logP close to 2.2, it leans toward lipophilicity, lingering in fats and oils. The presence of both methoxy and ethyl groups attached to a benzene ring shapes both reactivity and sensory profile. As with most phenolic compounds, 4-Ethylguaiacol shows moderate stability but can oxidize under harsh conditions, producing a range of byproducts.
When it comes to technical specifications, the industry relies on clear guidelines. Purity, moisture content, and identification through spectral methods define commercial lots. Labels prominently display batch number, production date, and storage suggestions since temperature swings or sunlight degrade quality. Material safety data sheets (MSDS) provide users with information on hazards and emergency measures. Anyone purchasing 4-Ethylguaiacol for food, beverage, or research expects transparency about impurities and potential allergens. Packaging—usually amber glass or high-grade plastic—protects the liquid from photodegradation. Reliable suppliers include a certificate of analysis, reassuring users about compliance with local and international standards.
Large-scale production of 4-Ethylguaiacol typically follows two main routes: extraction from natural sources or chemical synthesis. Extraction begins with lignin-rich plant material, since guaiacol and its derivatives abound in wood smoke and certain spices. More often, synthetic pathways dominate for reasons of cost and consistency. One common method involves ethylation of guaiacol using ethyl iodide or ethyl bromide in the presence of a strong base. This reaction pushes the ethyl group onto the aromatic ring, creating the desired substitution pattern. Catalysts, temperature control, and careful quenching minimize unwanted side products. Manufacturers refine the crude mix through distillation and column chromatography. For specialty markets, such as analytical standards, even more stringent purification steps—like high vacuum distillation—ensure the highest degree of cleanliness.
The reactivity of 4-Ethylguaiacol centers on its phenolic and methoxy functions. Both groups impact its behavior in oxidative or reductive environments. Under oxidative conditions, enzymes or chemical agents can convert 4-Ethylguaiacol into quinone-type structures, which sometimes exhibit altered aromas or biological activity. Alkyl and acyl substitutions at available positions provide a way to generate derivatives for sensory studies or novel fragrance applications. Through methylation or acetylation, chemists tweak volatility and persistence, opening fresh opportunities for use in flavor and scent design. In biological settings, selected bacteria and yeast can break down 4-Ethylguaiacol, connecting it to broader cycles of organic matter turnover. Modified versions—like brominated or chlorinated guaiacol derivatives—occasionally help in tracing metabolic pathways or developing bioactive molecules.
The literature uses several names for 4-Ethylguaiacol, depending on industry or context. Chemists refer to it as 4-Ethyl-2-methoxyphenol, which highlights its molecular structure. Some texts use the name β-Guaiacol or para-Ethylguaiacol. In food and beverage circles, it sometimes appears under flavor ingredient codes or trade names when listed in formulations. Synonyms allow clear communication between regulatory agencies, researchers, and industry. The variety of names comes from historical conventions and changes in IUPAC naming rules over time.
Handling 4-Ethylguaiacol in a laboratory or manufacturing facility calls for vigilance. Its moderate toxicity profile means gloves, goggles, and proper ventilation matter. Spills release a noticeable odor that lingers, which can alert staff to leaks. Though not highly flammable, storing it away from strong oxidizers limits risk. Food industry applications require further scrutiny: food-grade lots need to pass tests for heavy metals and residual solvents. Regulatory authorities—like the FDA and EFSA—set upper limits for concentrations in consumables, reflecting evidence that high levels affect product safety. Facilities document exposure levels to protect workers, and disposal must follow environmental rules, as phenolic compounds could affect aquatic systems. Warnings on containers ensure that only trained personnel have direct contact, reducing accidental exposure or contamination.
The place of 4-Ethylguaiacol spans a wide landscape. It stands out as a signature compound in barrel-aged wines, lending complex aromas prized by some and avoided by others. In the world of whisky, bourbon, and craft beer, producers either encourage or suppress its formation by controlling barrel conditions and microbial growth. Specialty food manufacturers blend it into flavors for sauces, meats, and smoke-scented products—always carefully balancing to avoid overwhelming the palate. Some perfumers use it to impart warmth or smoky facets in niche fragrances. In research, 4-Ethylguaiacol becomes a reference standard for quality control, supporting accurate assessment of flavor integrity. Its role as a microbial marker also supports studies on spoilage and fermentation dynamics, guiding better management techniques in food production and storage.
Scientific curiosity around 4-Ethylguaiacol keeps pushing boundaries. Food chemists continue looking for ways to distinguish between its natural and synthetic origins within complex mixtures, using isotopic and spectrometric tools. Advances in microbial genomics reveal how different yeast and bacteria strains produce or metabolize it, offering new strategies for fermentation tuning. With sensory science, researchers map interactions between 4-Ethylguaiacol and other flavor molecules—sometimes amplifying, sometimes masking key notes. Innovation in encapsulation and controlled-release technologies aims to stabilize this compound in finished products, protecting delicate aromas through tough supply chains. In the analytical lab, new protocols increase sensitivity, helping trace amounts get flagged before affecting consumer experience.
Toxicological studies give a measure of reassurance at common exposure levels. Testing in rodents shows low acute toxicity, but high doses can lead to irritation or organ effects. Regulatory reviews draw a line between beneficial flavor enhancement and potential hazards, especially in products consumed over a lifetime. Skin and eye contact prompts irritation for most people, underscoring the need for good laboratory habits. No clear evidence links 4-Ethylguaiacol with chronic toxicity or carcinogenicity at dietary levels. Research continues around metabolic products created in the body after ingestion or inhalation, with most findings to date indicating rapid breakdown and excretion. Still, responsible actors in food and beverage manage concentrations well below health thresholds, respecting the gap between optimal taste and safety.
Looking ahead, the story of 4-Ethylguaiacol seems far from over. Greater interest in craft beverages and artisanal foods will likely boost attention paid to the subtle details it brings. As winemakers and brewers seek differentiation, a deeper knowledge of how to coax or control its formation could give rising stars an edge. Analytical tools will only keep improving, helping producers dial in preferred aromatic profiles and reduce spoilage. Ongoing research into consumer perception might redefine what counts as a defect versus a selling point, shifting industry attitudes once again. Biotechnological advances could yield novel approaches to biosynthesis, perhaps unlocking greener, more sustainable routes to this storied flavor molecule. Amidst all this, the need for balance prevails: harnessing 4-Ethylguaiacol’s strengths without crossing the line into excess or harm.
Few people wake up thinking about 4-Ethylguaiacol, yet it comes up more often than you might guess, especially for fans of fine wine, specialty coffee, and unique whiskeys. In the world of flavors and aromas, this compound carries a lot of weight, shaping the experience of sipping a Pinot Noir or taking in the scent of a roasted brew. The story really begins with yeast and bacteria at work—4-Ethylguaiacol forms as part of a larger process of fermentation and aging, showing up as a marker of microbial activity in both food and drink.
My own time at various wineries taught me just how quickly a batch can shift from promising to flawed. 4-Ethylguaiacol plays a big part in that transformation. Its aroma, a blend of spicy, smoky, and sometimes medicinal tones, taps directly into the sensory side of things. In wine, 4-Ethylguaiacol often flies under the radar until it reaches a certain threshold. Beneath that point, it adds a layer of complexity—think toasted bread or bacon fat notes. Wine enthusiasts sometimes enjoy these nuances, especially in aged reds like Burgundy or Rioja. Push those levels too far, though, and the product soon veers into something closer to sticking plasters or overdone smokehouse, which rarely brings folks back for a second glass.
Coffee roasting also depends on modest traces of 4-Ethylguaiacol. Roasters use sensory evaluation to identify beans that yield the right aromatic profile. Lower concentrations might lead to positive reviews, with tasters picking up hints of clove or sandalwood. Over the years, quality control in specialty roasteries has leaned more on chemical analysis, giving labs a chance to flag lots before flawed beans reach supermarket shelves.
Brettanomyces yeast helps lead to the presence of 4-Ethylguaiacol in beverages. Vineyard managers and cellar teams go to great lengths to control that yeast—not to eliminate it entirely, but to keep its influence manageable. Oak barrels, common in both wine and whiskey production, create the right setting for Brett to thrive. Sanitation routines take on extra meaning since residual microbes love to hide in porous wood staves. In some breweries and cellars, sulfur dioxide serves as a line of defense. Used carefully, this additive keeps microbial blooms in check without stripping away too much of what makes a whiskey or wine unique.
Food scientists investigate 4-Ethylguaiacol for its use as a potential marker of spoilage. With advanced methods such as gas chromatography, labs break down a drink’s chemical makeup and use that data for quality assurance. The presence of this compound plays a prominent part in evaluating whether a batch can be sold, shipped, or needs further attention. The chemistry community continues studying its sensory impact, learning more about why some cultures accept funky notes as desirable and others flag them as defects.
In my time in kitchens and cellars, balance always seemed to win out over perfection. Instead of pushing for absolute purity or banishing wild elements, producers work with nature’s quirks to create flavors people won’t forget. By testing, cleaning, listening to tasters, and evolving with new research, they keep 4-Ethylguaiacol right where it belongs—on the fine line between complexity and flaw.
People who work with wine, whisky, or certain labs will recognize 4-Ethylguaiacol by its smoky, spicy smell. Found naturally in oak-aged drinks, it adds flavor in low amounts but quickly turns unpleasant above a certain threshold. In manufacturing or research, this compound arrives as a clear liquid with a strong aroma, and its chemical properties mean workers can’t treat it like common kitchen vinegar.
Back in grad school, routine tasks led to handling plenty of tricky chemicals—every bottle had a habit of surprising anyone too relaxed about safety. 4-Ethylguaiacol reminded me of phenolic substances with an aroma that clings to skin and gloves. Even in chemistry circles, a whiff would give the impression of scorched wood and old bandages. Nobody wanted to show up to lunch reeking of it.
The substance itself doesn’t jump out as acutely toxic, but that doesn’t mean people treat it like a harmless flavoring. On the skin, it can irritate pretty fast, and inhaling its vapors in a poorly ventilated space often leads to headaches or respiratory discomfort. Eyes burn with the mildest splash. Each safety data sheet treats exposure seriously: gloves, lab coats, and eye protection are musts.
The vapors linger more than many expect. I’ve seen colleagues underestimate the spread of strong-smelling compounds—once opened, their presence seems everywhere. Those unfamiliar with organic solvents sometimes develop a rash or start coughing if exposed for long.
A basic respect for well-characterized risks goes a long way. Records from the National Center for Biotechnology Information highlight skin and eye irritation, and wine-industry guidance emphasizes strict protocols. It’s often included in lists of workplace respiratory hazards, which brings back memories of poorly ventilated undergraduate labs with little extraction. Workers elsewhere—flavor labs, distilleries, even environmental researchers—share similar stories.
Exposure limits for this chemical don’t receive global regulation, since it’s not produced in vast quantities compared to solvents like acetone or toluene. But its effects stack up all the same in small spaces.
Moving forward, small changes make big differences when handling this stuff. Start with working in a fume hood—not just a cracked window. Regular nitrile gloves hold up just fine but swap them out if any splashes occur, since phenolic chemicals soak through over time. Unused to the smell? Alert co-workers before opening a bottle, since a prep room can fill up quickly.
Label every container, even for quick transfers. Spills should go straight onto absorbent pads and into hazardous waste bins rather than down the sink. Companies really help by training all staff, not just chemists, to identify phenolic odors and understand exposure symptoms.
Sooner or later, everyone who works with strong-smelling organics learns that following safety rules isn’t just about compliance—it's about avoiding irritation, lost workdays, and bad habits that can stick. 4-Ethylguaiacol brings flavor to drinks, but nobody enjoys that spice on their hands or in their lungs.
Sticking to gloves, goggles, and good ventilation makes this compound manageable, letting professionals focus on production or research instead of hospital visits.
4-Ethylguaiacol stands out for those who pay close attention to taste and aroma, especially in food and drink circles. Its smoky, spicy character often signals a familiar note in aged wines, whiskey, and even roasted coffee. The way this compound behaves at the basic physical level ties directly into its role in these products.
Held in a clear vial, 4-Ethylguaiacol looks like a colorless to pale yellow liquid. Pour a few drops on a piece of paper, and the aroma escapes fast—campfire, clove, or smoked bacon, depending on who’s sniffing. That strong scent isn’t just for show: it hints at a lower molecular weight (the number sits around 166.22 g/mol), marking the compound as fairly volatile. That volatility makes it move quickly into the air, impacting taste and nose even at low concentrations. Pick up a bottle of smoky Syrah, take a sniff, and there’s a good chance some portion of the impact comes from this exact behavior.
Anyone working in a lab notices that 4-Ethylguaiacol boils at roughly 240°C. That’s tame for small aromatic compounds, so it stays liquid under most storage conditions. It doesn’t freeze until around -27°C, meaning even a chilly cellar won’t turn it solid. Its moderate polarity (thanks to the methoxy and hydroxyl group) gives it some water solubility, though it prefers to hang out in organic solvents like ethanol or diethyl ether. In winemaking, this means it can shift easily between water-rich grape juice and the alcohol-dominant wine, never quite disappearing or becoming stubbornly insoluble.
Each time I’ve worked with 4-Ethylguaiacol in an analytical lab, the room fills up quickly with that sharp, smoky aroma. Those volatile organic molecules can be overpowering—wearing gloves and working in a fume hood isn’t just a habit, it’s a requirement. At low concentrations, it doesn’t pose a big health risk, but higher doses start to irritate the eyes and nose. Anyone running flavor trials or quality control for the beverage industry knows that careful handling means more than just following rules—it keeps the day manageable when subtle off-flavors or excessive smokiness must be avoided.
People sometimes ignore the nuts and bolts of a flavor compound, thinking those boil points or densities belong to chemists only. Yet those properties steer how 4-Ethylguaiacol spreads through a batch of wine, influences storage life, and even affects shipment and labeling under regulations. A compound with a strong aroma at low concentration influences blending decisions for barrels, coffee roasters’ timing, and how a final bottle ends up tasting in your glass. Brewers and vintners often use gas chromatography to spot traces because even 5 micrograms per liter can tip a product from unique to unacceptable.
Keeping unwanted earthy or medicinal notes out of food and drink often means tracking down the source of 4-Ethylguaiacol or its close cousins. This usually leads back to spoilage yeasts like Brettanomyces in barrels or tanks. Winemakers use careful cleaning, sulfite additions, and good temperature control to hold levels in check. As much as the physical properties help this molecule escape into the air, those same characteristics allow precise tracking and intervention: headspace analysis picks up its volatility, and chromatography picks out its chemical fingerprint. In my time working side by side with winemakers and lab techs, every trial, every adjusted barrel, and every analytical run starts with respecting what these properties reveal.
4-Ethylguaiacol doesn’t show up on most people’s grocery list, but it holds a real spot in industries that touch everyday life. This compound gives wine and whiskey their signature smoky, spicy notes, but pure 4-Ethylguaiacol is no friend to careless handlers. It’s flammable, carries a strong odor, and can irritate skin or eyes. Whenever businesses deal with it, a practical approach keeps both workers and products safe.
Most workplaces where safety matters store 4-Ethylguaiacol in airtight glass bottles or stainless steel drums. These materials don’t react with the compound and help contain its powerful scent. Cooler temperatures help slow down any unwanted reactions. Direct sunlight isn’t just bad for milk; it can break down this compound faster than you’d expect. That’s why smart warehouses sit away from windows and heat sources. Extra care means using flame-proof cabinets to keep accidents in check.
Labels matter as much here as any other part of the supply chain. Missing or faded labels leave workers guessing, and mistakes get costly. A well-run operation brings in regular checks, taking time to look for corrosion or leaks. Leaks don’t just cost money—they invite risks nobody needs in a chemical storage room.
Shipping any flammable chemical isn’t something to take lightly. Trucks, trains, or ships carrying 4-Ethylguaiacol use UN-certified packaging that holds up against rough bumps or surprise weather changes. Tightly sealed drums or jerricans stand up to bumps on the road. Drivers or rail operators keep paperwork in order, with hazard labels clearly posted. This keeps emergency crews informed and helps avoid confusion.
People often think a quick drive will fix any delay. That’s not the case with flammables. Long delays or detours leave barrels sitting in hot sun on an asphalt lot, raising risk. Smart logistics means tracking cargo movement in real time. Companies with strong records keep delivery routes tight and avoid risky shortcuts just to shave some time off their schedules.
Accidents often trace back to a broken link in the safety chain: a rusty cap, late check, or poorly chosen storage room. Compounds like 4-Ethylguaiacol need teams on the lookout for small problems before they become big ones, especially since regulatory fines for spills can reach tens of thousands of dollars, not to mention damage to reputation. A strong workplace culture supports workers in calling out leaks or errors, and managers who listen make both the workplace and the product more reliable.
Training pays off. People learn to spot early warning signs, how to handle small spills, and when to pick up the phone for help. Hazmat plans on paper matter less than drills on the warehouse floor. Good managers walk the floor themselves, showing respect for workers and the risks of the job.
Practical changes can make a world of difference. Invest in quality containers and tracking tools. Use sensors for heat and vapor build-up—not just in the biggest warehouses, but even at smaller storage sites. Share incidents with the team, not just the legal department. Treat near-misses as valuable lessons, not something to hide away.
The science behind 4-Ethylguaiacol brings benefits to flavor and fragrance. Keeping this chemical safe on the shelves and the roads asks for more than rules. It calls for clear thinking, strong teamwork, and respect for both people and material. Companies that earn trust put these lessons to work every day.
People working in food science or winemaking hear a lot about 4-ethylguaiacol. Some know it as one of those molecules that smack right into your nose, bringing the aroma of smoked bacon, spice, or something like old leather. Others look for it because it changes wine, whiskey, or even beer — sometimes in a good way, sometimes not. I remember standing in a winery outside Paso Robles, talking with a vintner about how Brettanomyces yeast can pump out so much of this stuff that a beautiful barrel turns into a barnyard in a bottle. That conversation stuck, because it drove home how a little chemistry decides flavor and value. Now, more people want to get their hands on pure 4-ethylguaiacol—not to ruin drinks, but to study, to train palates, or to calibrate equipment.
Unlike table salt or vanilla extract, you won’t spot 4-ethylguaiacol on supermarket shelves. The chemical supply chain splits between labs, businesses, and certain online outlets that vet customers and enforce safety rules. Companies like Sigma-Aldrich and TCI Chemicals list it in small bottles, often as part of flavor standards or chemical reference kits. I’ve seen prices swing from $50 to hundreds of dollars, depending on purity and volume. Retail restrictions come from laws governing who can buy hazardous chemicals. Shipping this stuff isn’t simple: it triggers paperwork and age verification, sometimes even business licenses.
This kind of setup can frustrate a researcher or aspiring craft distiller. It can also protect ordinary consumers from casual, unsafe experimentation. 4-Ethylguaiacol isn’t considered a super-dangerous compound, but swallow enough or spill it in your eyes, and it’ll put you out of commission. For that reason, the major suppliers run checks to verify your background. You’ll get asked about your lab, your project, and your qualifications. Anyone selling without those checks probably isn’t reputable, and there’s a risk of counterfeit or mislabeled product. Amazon and eBay sometimes pop up with listings, but reliability drops fast outside official lab supply channels.
In my consulting work, wine producers often ask if there’s an easier or more affordable way to get compounds like 4-ethylguaiacol. I have to remind them about the regulatory environment. In the United States and Europe, safety agencies like OSHA and the European Chemicals Agency monitor distribution of volatile organics. Transport rules force companies to declare the chemical and demonstrate proper storage. Consumer safety trumps speed or convenience, at least for now.
If you want to order a small quantity for research or training, reach out directly to lab supply firms. Have your documentation ready. University-backed requests go through procurement offices. For industry, a business account speeds up the process. Anyone working alone should link up with a local university, makerspace, or food-quality lab to piggyback on their supply chains.
Some winemakers create “house standards” by collecting Brett-heavy wines as natural reference points. That works for tasting but not instrument calibration, which needs pure chemical standards. Sensory training kits sold by companies specializing in wine faults offer diluted versions of 4-ethylguaiacol, with built-in safety guides and dosing instructions. These kits simplify sourcing and limit exposure by keeping concentrations low and volumes small. That approach strikes me as practical — it protects novices and meets most needs outside advanced research.
Transparency matters. Whatever route you take, confirm that any 4-ethylguaiacol comes with a certificate of analysis from a reputable supplier. This documents the source, purity, and batch, which keeps the research legit and guards against surprises. Buying direct from chemical distributors isn’t as easy as shopping online, but it’s the only way to get something clean, safe, and traceable. It doesn’t just keep you out of trouble — it ensures you’re working with what the label says, no mystery included.
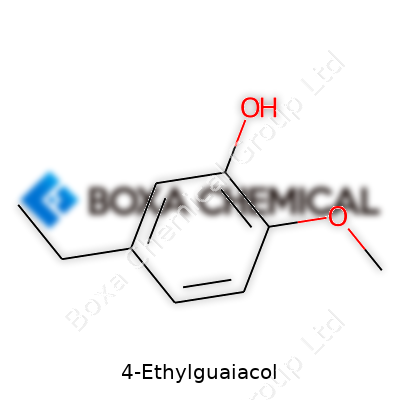
| Names | |
| Preferred IUPAC name | 2-Ethyl-6-methoxyphenol |
| Other names |
4-Ethyl-2-methoxyphenol 4-EG p-Ethylguaiacol |
| Pronunciation | /ˈfɔːr ˌɛθ.ɪl.ɡwaɪˈæk.ɒl/ |
| Identifiers | |
| CAS Number | 2785-89-9 |
| Beilstein Reference | 1368730 |
| ChEBI | CHEBI:28682 |
| ChEMBL | CHEMBL552272 |
| ChemSpider | 12392 |
| DrugBank | DB04261 |
| ECHA InfoCard | 100.013.953 |
| EC Number | 4.1.1.114 |
| Gmelin Reference | 787805 |
| KEGG | C12166 |
| MeSH | D000068409 |
| PubChem CID | 8090 |
| RTECS number | EZ8575000 |
| UNII | K4I1X32T2B |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C9H12O2 |
| Molar mass | 152.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | phenolic; clove-like; medicinal |
| Density | 1.037 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.93 |
| Vapor pressure | 0.19 mmHg (at 25 °C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 10.38 |
| Magnetic susceptibility (χ) | -71.0e-6 cm³/mol |
| Refractive index (nD) | 1.536 |
| Viscosity | 1.204 mPa·s (25 °C) |
| Dipole moment | 1.81 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -309.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4295.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P362+P364, P370+P378, P403+P235, P405, P501 |
| Flash point | Flash point: 113 °C |
| Autoignition temperature | 415 °C |
| Explosive limits | Explosive limits: 1.3–8.3% |
| LD50 (median dose) | LD50 (median dose): 990 mg/kg (rat, oral) |
| NIOSH | EW3675000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 ppm |
| Related compounds | |
| Related compounds |
Guaiacol 4-Vinylguaiacol 4-Methylguaiacol Eugenol |