4-Chlorophenol has an interesting backstory, tracing its roots to the early days of synthetic chemistry. Early 20th-century chemists eyed phenolic compounds as building blocks for dyes, disinfectants, and even pharmaceuticals. As chlorination techniques matured, introducing a chlorine atom to the phenol ring brought new functionality. Farmers, manufacturers, and even municipal workers quickly found value in these alterations, tapping into the compound’s antimicrobial punch and stability. Over the decades, 4-Chlorophenol carved out its own place, nudging aside some natural phenols in industrial applications thanks to its chemical stubbornness and punchy activity against microbes.
The chemical structure of 4-Chlorophenol—just a chlorine atom substituted at the para position on a phenol ring—might look unassuming, but it brings a unique mix of reactivity and persistence. This compound heads into everything from disinfectant formulations, to resin intermediates, to niche pharmaceuticals. In my experience reading chemical inventories, I rarely see a specialty chemical catalogue without one or two grades of 4-Chlorophenol on offer. Companies prize it for its high purity and consistent melting point, knowing that even a small deviation can sink a batch or trigger storage issues. The ingredient list on some legacy wood treatments and cleaner products also betrays its presence.
4-Chlorophenol drops out of solution as white or slightly off-white crystals, releasing a sharp, medicinal odor that veterans in the lab always recognize. With a melting point hovering near 43°C and a boiling point past 210°C, it holds up in many tough process conditions. The chemical resists mild acids and bases, but strong oxidants and alkali will eventually break it down. Solubility sits low in water, but organic solvents like ethanol or ether do a far better job dissolving it. The crucial thing about this compound: it clings to surfaces and rarely evaporates, which gives it staying power as both a biocide and a challenge for remediation.
Anyone handling or buying 4-Chlorophenol needs a tight spec sheet. Industrial material usually offers a purity above 99%, with known limits for related isomers and heavy metals. I’ve seen some low-grade stocks floating around with yellowing from impurities; savvy buyers run GC or HPLC tests to keep their processes predictable. Packaging labels must feature the UN number (2021), GHS hazard diamonds, and details about reactivity and toxicity. Proper batch numbers and QA signoff also matter for tracking and liability. The labeling doesn’t just inform—it helps manage accidents, recalls, or audits from health agencies.
Large-scale production of 4-Chlorophenol relies on direct chlorination of phenol under controlled temperatures and catalysts. The goal focuses on pushing the substitution to the para position and minimizing ortho-isomers. Some manufacturers even employ iron(III) chloride or copper catalysts to sharpen selectivity. As new methods pop up in academic journals—like ultrasound-assisted or micellar-phase chlorination—producers experiment to cut energy costs or reduce unwanted byproducts. Wastewater streams from manufacturing sites often need careful treatment since chlorinated phenols pose a persistent environmental risk, echoing decades of growing pains in the chemical industry.
4-Chlorophenol’s reactive sites drive its value. Nucleophilic substitutions, such as conversion to amines or ethers, become more possible with a good leaving group like chlorine. I’ve seen companies substitute the chlorine for nitro- or amino groups, spinning the molecule into dyes or agrochemical bases. The phenolic OH can undergo etherification or acylation, leaning into perfume ingredients or plastic softeners. Chemists chasing niche molecules appreciate 4-Chlorophenol’s predictable reactivity and ability to anchor more complex structures, especially once they figure out how to control side products and reaction times.
Industry and academia know 4-Chlorophenol by a laundry list of names—p-Chlorophenol, para-Chlorophenol, and sometimes just PCP. Commercial products run under trade names ranging from Dow’s Chloro-Cid to French variants like Phénol para-chloro. Researchers sorting through historic patents often find the same molecule listed under slightly varied brand identities, feeding confusion for newcomers. Standardization efforts have gained steam recently, but walking older sites or reading legacy documents still uncovers synonyms not used much these days.
People coming into contact with 4-Chlorophenol can't ignore its hazards. Even trace skin contact can burn or cause allergic reactions in sensitive folks. I remember colleagues comparing it to formaldehyde for its tendency to dry out skin and breathe stinging vapors. Factories processing the material must run local exhaust ventilation, splash-proof PPE, and real-time vapor monitoring. OSHA guidelines and the EU’s REACH framework both restrict workplace exposures. Storage means acid-resistant drums, cool rooms, and doors that don’t jam in emergencies. Every accident report I have come across underscores one point: this is not a compound to handle casually or with shortcuts.
Wood preservation stands out as a classic use, especially in treating beams against termites and molds. Municipal water treatment plants used to lean on 4-Chlorophenol as a biocide, though concerns about environmental persistence have pushed the industry toward less tenacious alternatives. In the pharmaceutical world, the compound serves as a platform for synthesizing antiseptics and certain anesthetics. Dye manufacturers tweak its structure to yield stubborn shades that don’t fade in sun or rain. Testing laboratories also spike samples with 4-Chlorophenol as an internal standard, trusting its stability through rigorous analysis runs.
R&D teams, especially in Asia and Europe, continue to look for cleaner synthesis routes and new downstream products. I’ve read studies experimenting with enzyme-catalyzed transformations to replace old-school metal catalysts, while others work to boost yield or limit waste. Novel derivatives, like fluorinated or alkylated analogues, fill journal pages with fresh compound libraries targeting stronger antimicrobial or pest-control performance. Academic groups keep hunting for ways to break down 4-Chlorophenol in contaminated groundwater, relying on positive lab data to shape future site remediation policies.
Toxicologists have followed this molecule for decades, noting it’s more than just a skin hazard. Rats exposed through inhalation or ingestion display liver and kidney damage, with dose dependence showing up in blood and urine markers. Long-term studies flagged a possible cancer risk in animals, and the EPA added it to the hazardous substances list. Some environmental advocates push for tighter wastewater controls, pointing to groundwater contamination in areas around historic wood-treating plants. Analytical chemists in public health agencies often test for metabolites of 4-Chlorophenol during routine screenings of fish, soil, and even breast milk samples.
Future prospects for 4-Chlorophenol look mixed. Demand will likely drop in major municipal and consumer sectors, as substitutes with lower toxicity edge in and stricter global regulations clamp down. Specialty chemical and pharmaceutical firms probably won’t abandon it—its scaffold fits too well in processes for dyes, drugs, and laboratory reagents. Cleaner manufacturing and more effective remediation technologies could ease some concerns about persistence. Startups dabbling in biodegradable pesticides and green solvents might revisit modified versions or use phenol chlorination data to avoid old mistakes. The next generation of scientists faces a dual job: balance this compound’s decades-old usefulness with urgent environmental and health realities that simply won’t go away.
4-Chlorophenol shows up in a lot of places people don’t usually see. It’s a chemical made by swapping out one hydrogen atom in a phenol ring with a chlorine atom. That little change packs a punch, since it affects reactivity and makes this compound useful for many applications across different fields. I’ve noticed that chemicals like this one often serve as building blocks—once you get used to their scent (sharp, even unpleasant), you don't forget them in a hurry.
Out on farm fields, 4-Chlorophenol plays a behind-the-scenes role in helping crops stay healthy. Manufacturers use it to produce pesticide and herbicide molecules. For example, 2,4-dichlorophenoxyacetic acid (2,4-D), a common weed killer, comes from reacting this chemical at specific positions on the ring. Without 4-Chlorophenol, these formulations would cost more, and alternatives sometimes carry higher eco-toxicity. From experience talking with farmers, I’ve seen that reliable crop protection remains a constant focus, and chemicals like these keep fields producing bushels and tons, not just sprigs.
Hospitals and clinics have their share of uses for 4-Chlorophenol, especially in the world of antiseptics. The compound often ends up as a raw material in the creation of disinfecting agents and antiseptics. Chloroxylenol, found in many household antiseptics, comes to life through a process starting with 4-Chlorophenol. In some countries, this means hospital staff rely on it to clean wounds and equipment, especially in places where more advanced solutions may not be available or are too costly. It might seem like a small link in the supply chain, but medicine cabinets and first-aid kits around the world still draw on this chemical’s legacy.
Textile processing fascinates me because so many colors and textiles pass through chemical reactions few people think about while picking clothes off a store rack. 4-Chlorophenol makes it into pigment, dye, and resin production, serving as a key ingredient in synthesizing azo dyes and epoxy resins. These materials turn up in everything from bright shirt fabrics to glossy coatings on appliances. Without sturdy intermediates like 4-Chlorophenol, industrial chemists spend more money making the color wheel spin. History shows that cheap access to chemicals like this has kept down the cost of everything from blue jeans to plastic kitchenware.
I always worry about what happens to chemicals after their useful life ends. 4-Chlorophenol brings some risk. It can harm aquatic life, persist in soil, and sometimes enter water supplies through manufacturing waste. News keeps coming about contaminated groundwater near chemical plants. Dealing with this problem means stronger rules on chemical disposal and better water treatment technology. My neighbors in industrial towns talk about learning from earlier mistakes—asking for stricter monitoring and reporting requirements. Green chemistry groups keep pushing for safer, less toxic alternatives, and some research promises plant-based synthesis pathways with less pollution.
4-Chlorophenol demonstrates the push-pull balance between industry and the environment. It keeps things growing on farms, guards against infections, and colors the world, but also raises questions about health and sustainability. Smarter regulation, newer production routes, and better cleanup technology all shape how long this compound keeps its place in so many industries. The challenge is trading none of the benefits while cutting down on harm, a goal chemists and consumers both share, whether they realize it or not.
4-Chlorophenol is more than just a chemical name from a textbook. Commonly found in disinfectants, antiseptics, and wood preservatives, it carries serious risks for workers in research labs, manufacturing, and waste treatment plants. This compound gives off a strong odor and, on contact, can cause burns, eye damage, and respiratory trouble. Long-term exposure brings even steeper consequences—liver and kidney damage, or even cancer. Workers deserve to know what they’re dealing with, not just from a safety data sheet, but from honest conversations about the dangers in the air and on their skin.
Nitrile or neoprene gloves won’t last forever against harsh chemicals, but they stand up better than latex. I once watched a colleague hurriedly grab thin gloves before handling chlorophenol, figuring they’d be “good enough,” only to end up with burns. Thick gloves, safety goggles, face shields, and a sturdy lab coat make a world of difference. Because this stuff can vaporize even at moderate temperatures, a respirator with organic vapor cartridges keeps lungs out of harm’s way. Open windows, work inside a fume hood, and keep skin covered head to toe. Don’t rush—double check your gear every time.
Ventilation is not a luxury—it’s the barrier between a safe workspace and a toxic one. Anyone who has ever gotten a whiff of chlorophenol knows the value of a well-designed fume hood. Every step, from pouring to mixing, should happen inside proper ventilation. A spill on a crowded workbench can spread fumes in seconds. Cleanliness must be a shared value. Remove contaminated clothing right away. Shower thoroughly, disposing of wash water safely, far from public drains or local streams. Chemical-resistant aprons and closed shoes stop splashes from turning into a trip to the ER.
No one expects a beaker to drop, but accidents happen. Absorbent pads, neutralizing agents, and spill kits sit within arm’s reach for a reason. Knowing how to mop up and isolate a spill can stop a minor accident from becoming a major disaster. Training cannot be skimmed over—safety drills build confidence, turning panic into action when it matters most. People learn best from experience, but the classroom comes first. Rehearse those emergency showers and eyewash stations. Test them, so everyone knows the drill when seconds count.
Once work wraps up, the substance won’t just disappear. Pouring leftover 4-chlorophenol down the drain is unthinkable; it poisons water systems and harms wildlife. Store waste in airtight, clearly labeled containers. Connect with hazardous materials teams for proper disposal—cutting corners creates a ticking time bomb for the next team. In my own lab, mistakes in labeling cost us time, but careless disposal would have cost us so much more. Documentation—clear, honest, and updated—protects everyone who comes after you.
The talk about personal responsibility goes beyond checklists and PPE. People watch each other. Peer reminders, frequent training updates, and honest reporting of near-misses all build trust. Complacency creeps in when routines set in, so regular safety meetings bring fresh eyes to old habits. 4-Chlorophenol demands more than respect—it asks for vigilance in every step, from preparation to disposal. The team stays safe when everyone treats these precautions like second nature.
Anyone who’s worked even briefly in a laboratory or environmental testing space will bump into aromatic compounds like 4-chlorophenol. The chemical formula for 4-chlorophenol is C6H5ClO. That boils down to six carbon atoms, five hydrogens, a single chlorine atom, and one oxygen.
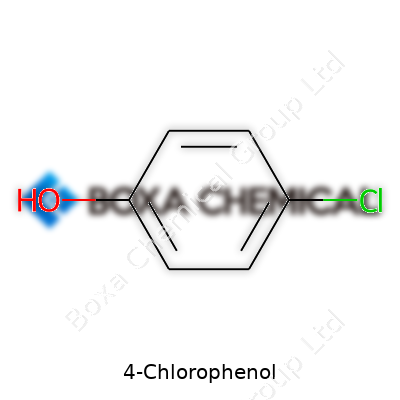
4-Chlorophenol's shape always brings me back to organic chemistry lectures—it's essentially a benzene ring with a hydroxyl (–OH) group and a chlorine (Cl) smack across from each other. That kind of arrangement, known as the para position, means the –OH sits on one carbon and the chlorine on carbon number 4 if you start counting from the –OH. Chemists often draw it as a six-sided ring with alternating double bonds, tacking the –OH and –Cl onto opposite sides. This unique shape affects everything from how it dissolves to how toxic it is.
This isn’t just some abstract molecule living on a whiteboard. 4-Chlorophenol makes its way into a surprising number of places. You’ll find it in water treatment plants as a degradation product from pesticides and disinfectants. Anybody living close to industrial zones knows odd smells from treated wood, and compounds like this often play a part.
Outside factories, 4-chlorophenol crops up in the monitoring reports of rivers and groundwater. One thing I’ve seen is how fast stakeholders react once this compound pops up in a water sample. Regulatory agencies set pretty tight rules because chlorinated phenols have a reputation for being toxic to both people and wildlife. Even low doses can cause irritation, and some studies tie long-term exposure to potential health problems.
Scientists, environmentalists, and regulators pay extra attention to 4-chlorophenol for good reason. Even at small concentrations, it smells sharp and medicinal, which makes it easy to notice. You might not see it, but once it’s present, you can bet that fish, insects, and plants are getting the worst end of the bargain. I remember seeing reports of fish kills traced directly to chlorophenol spills from local mills—fish just floated up, and it took weeks to recover the local creek.
One fact that surfaces often: 4-chlorophenol doesn’t break down all that easily. Sunlight and certain bacteria help, but it lingers, especially when released into groundwater. From a public health perspective, letting compounds like this spread leaves communities at risk. Groups that advocate for stricter testing—especially among vulnerable populations—aren’t just being picky. It’s about making sure the air and water stay safe for everyone.
It’s not all bad news. Newer cleanup approaches, such as advanced oxidation and improved degradation by engineered microbes, offer ways to trim down levels of 4-chlorophenol in contaminated sites. Investing in technologies for early detection goes a long way—smarter sensors mean faster responses. And, as someone who’s spent long hours with spectrometers and chromatography columns, I can vouch for the difference even small process changes make over years of monitoring.
Consumers have power in this story too. Supporting products and processes that minimize the use of chlorinated chemicals means fewer risky byproducts downstream. It takes a mix of smart chemistry, clear regulations, and active consumers to keep persistent pollutants like 4-chlorophenol in check.
Someone working in a lab or industry probably comes across 4-Chlorophenol more often than most. This chemical turns up in disinfectants, herbicides, and sometimes in pharmaceuticals. Its strong smell hangs in the air, catching attention even before reading a label. But it's more than just a sharp scent—4-Chlorophenol irritates skin, harms eyes, and triggers respiratory symptoms. Community health studies and EPA documents point out its toxicity to aquatic life, adding environmental worries that shouldn’t just get shrugged off.
A bottle of 4-Chlorophenol never belongs on a cluttered bench or somewhere that kids or pets wander freely. I keep it in a cabinet meant for corrosive or toxic chemicals, behind a locked door, with clear signage. The container matters too: no cracked glass, no rusty cap, no makeshift lids. Polyethylene or amber glass bottles with tight seals guard against leaks and accidental vapor release.
Room temperature storage works well, but keep that spot out of direct sunlight and away from sources of heat or open flame—4-Chlorophenol catches fire more easily than many realize. Don't stash it above eye level, and always separate acids, bases, and oxidizers in different compartments to avoid nasty chemical reactions if a spill happens.
Large-scale operations bring more risk. Secondary containment trays help catch leaks before they spread. Proper ventilation, ideally with a dedicated fume hood or an exhaust system, keeps vapors from building up. Writing dates and names on containers sounds old-fashioned, yet it prevents confusion and accidents down the road.
Nobody should pour leftover 4-Chlorophenol down a drain. Municipal water facilities rarely handle this pollutant; it can slip through and contaminate rivers, lakes, and drinking water. Toxic lingers on long after someone turns on the tap. Instead, place unwanted chemical in its original container, seal it tightly, and label it. Local and federal rules call for sending it to a licensed hazardous waste site. EPA and OSHA lay out clear disposal regulations, and ignoring those rules risks both fines and harm to the community.
Labs and companies should set up regular hazardous waste pickup schedules. Every batch of waste gets documented with a manifest. That paperwork means chemicals end up tracked through every step. City-run household hazardous waste events offer a good option for individuals or schools. Never mix 4-Chlorophenol with other disposables. Reactive chemicals in garbage or the sewer grate can turn toxic waste into dangerous gas or create a fire.
On rare occasions, chemical treatment or incineration might come up as options, but nobody should try these processes in their garage or backyard. Incinerators built for hazardous waste run at higher temperatures, use scrubbers, and protect air quality.
Training and clear instructions beat fancy technology every time. Workers need real, hands-on education on how to handle gases, liquids, and solids like 4-Chlorophenol. Good signage, inventory control, and emergency plans lay the groundwork for safe practices. Every facility should keep spill kits and protective gear within arm’s reach.
Both industry and individuals improve the odds by asking tough questions. Does this process need 4-Chlorophenol, or can a safer chemical take its place? Safer substitutions exist in some cases, and reducing reliance on legacy chemicals often pays off.
Storing and tossing out hazardous chemicals isn't just a technical job—it's a commitment to health, safety, and clean water for everyone. Keeping things simple, honest, and precise changes attitudes, not just rules.
The first time I heard of 4-chlorophenol, I was standing on the factory floor, watching workers in baggy overalls move barrels with clear hazard labels. This chemical shows up a lot in industries that deal with disinfectants, wood preservatives, and dyes. Its sharp, sickly odor lingers in the air long after spills. Workers keep gloves on, but even the best safety routines offer limited comfort if there’s a leak or a broken mask. In my experience, a splash or two on skin can bring burning, redness, and rashes that linger for days.
Inhaling fumes from 4-chlorophenol can do a lot more than just make someone cough. Headaches set in first, followed by a weird dizziness that blankets everything in brain fog. After an accidental spill in a poorly ventilated storeroom, I saw a co-worker clutching his stomach. Later, nausea became so bad he couldn’t finish his shift. Coughing and a scratchy throat seem common for people handling this stuff day after day. OSHA warns that chronic inhalation can lead to damage in the upper respiratory tract and even lungs—definitely not something to shrug off. Recent studies from the National Institute for Occupational Safety and Health paint a clear picture: regular exposure, even at low levels, may cause tissue inflammation, asthma-like symptoms, and trouble with basic breathing.
After a year watching people work with 4-chlorophenol, I started hearing more complaints. Muscle weakness, fatigue, and problems with memory showed up even outside work hours. The skin, it turns out, isn’t the only target. This chemical can sneak in through cuts and pores, then stress out the whole body. Scientists in toxicology journals often list liver and kidney damage as possible outcomes after prolonged contact or inhalation. The Environmental Protection Agency points out that long-term health effects can include an increased risk of certain cancers. Chlorinated phenols, including this one, break down slowly inside the body and the environment. That slow decay means effects can build up over years, making early symptoms easy to dismiss.
It’s not only a workplace issue. 4-chlorophenol likes to show up near manufacturing plants and dumpsites. People living nearby sometimes notice an odd taste in their tap water. In towns close to chemical processing plants, I’ve seen neighbors worry about skin irritation or breathing trouble after a heavy rain. The CDC warns that this pollutant contaminates ground and drinking water far too easily. I’ve followed news reports where contaminated wells forced whole neighborhoods to rely on bottled water for months. The risks for children and the elderly, who are more sensitive to environmental toxins, keep community groups pushing for regular monitoring and stronger cleanup efforts.
Staying safe around chemicals like 4-chlorophenol means advancing safety gear and updating policies. Employers need to invest in proper training and personal protective equipment so workers don’t leave the plant with more than just a day’s pay. Engineering controls—local exhaust systems, sealed containers—help keep fumes where they belong. I recommend regular air quality checks, because you can’t fix what you don’t measure. For the public, stronger environmental rules and better spill response help keep contaminated water from reaching homes. Organizations like OSHA and the EPA have useful guidelines, but constant vigilance is key. After all, safety in communities and on jobsites only grows when people push for honest conversations about real risks.
| Names | |
| Preferred IUPAC name | 4-Chlorophenol |
| Other names |
p-Chlorophenol 1-Chloro-4-hydroxybenzene 4-hydroxychlorobenzene p-hydroxychlorobenzene |
| Pronunciation | /ˌklɔːr.oʊ.fiˈnɒl/ |
| Identifiers | |
| CAS Number | 106-48-9 |
| 3D model (JSmol) | `4-chlorophenol|CC1=CC=C(C=C1)Cl|JSmol` |
| Beilstein Reference | 1209220 |
| ChEBI | CHEBI:15938 |
| ChEMBL | CHEMBL1399 |
| ChemSpider | 761 |
| DrugBank | DB14015 |
| ECHA InfoCard | 100.003.160 |
| EC Number | 4.99.61 |
| Gmelin Reference | Gmelin Reference: 8041 |
| KEGG | C01416 |
| MeSH | D002767 |
| PubChem CID | 6987 |
| RTECS number | SN4300000 |
| UNII | C059248CMM |
| UN number | UN2020 |
| Properties | |
| Chemical formula | C6H5ClO |
| Molar mass | 128.56 g/mol |
| Appearance | Colorless to pale yellow solid |
| Odor | aromatic phenolic odor |
| Density | 1.306 g/cm³ |
| Solubility in water | 20 g/L |
| log P | 2.39 |
| Vapor pressure | 0.4 mmHg (25 °C) |
| Acidity (pKa) | 9.38 |
| Basicity (pKb) | 9.38 |
| Magnetic susceptibility (χ) | -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.553 |
| Viscosity | 2.68 mPa·s (25 °C) |
| Dipole moment | 1.69 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 129.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -106.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3828.9 kJ/mol |
| Pharmacology | |
| ATC code | D08AE01 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes severe skin burns and eye damage; harmful to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS05, GHS06 |
| Signal word | Danger |
| Hazard statements | Hazard statements: H302, H312, H315, H318, H332, H341, H373, H400 |
| Precautionary statements | P260, P264, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P330, P337+P313 |
| NFPA 704 (fire diamond) | 3-2-0-A |
| Flash point | 79°C |
| Autoignition temperature | 605°C |
| Explosive limits | Not found |
| Lethal dose or concentration | LD50 oral rat 450 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Chlorophenol: "130 mg/kg (oral, rat) |
| NIOSH | C6H5ClO |
| PEL (Permissible) | 5 ppm |
| REL (Recommended) | 2 mg/L |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Phenol 2-Chlorophenol 3-Chlorophenol 2,4-Dichlorophenol |