Chemists have shaped the story of 4-Chloroacetylcatechol across decades of research aimed at tailoring aromatic compounds for specific functions. Early work in the mid-20th century focused on catechol derivatives, and the addition of a chloroacetyl group showed promise in both reactivity and biological potential. Large-scale production grew as demand increased for reliable intermediates in pharmaceutical and agrochemical applications. By the end of the last century, this compound earned a seat at the table for pivotal synthetic methods, thanks to available chlorinating agents and established acetylation techniques. The evolution didn’t just bring new chemical pathways, but also prompted deeper studies into its applications and toxicity, especially in regulated industries.
4-Chloroacetylcatechol provides a building block for many chemical processes—think pharmaceutical intermediates, specialty polymers, and selective pesticides. Its structure carries both an electron-donating catechol core and a reactive chloroacetyl group. These functional groups attract researchers who need fine-tuned behavior for organic synthesis. Small and mid-sized chemical companies place bulk orders, not just because of its versatility but also for consistency in batch-to-batch quality, which matters in drug production pipelines. Most labs store it in tightly sealed glass bottles, away from light and heat, since its chemical integrity determines end-product performance.
4-Chloroacetylcatechol stands as an off-white to faint beige crystalline solid. Unlike more volatile chemicals, its melting point hovers in the upper range—often above 100°C—which gives some stability during handling and transportation. Its solubility favors polar solvents, such as dimethyl sulfoxide and acetone, while proving less cooperative with nonpolar solvents. With both hydroxyl groups on the aromatic ring and a chloroacetyl group bonded to the molecule, this compound brings both hydrophilic and hydrophobic character. Handling it always reminds chemists of its moderate volatility and the pungency of the chloroacetyl group, prompting swift attention to ventilation.
Producers of 4-Chloroacetylcatechol mark product labels with purity usually not less than 98%, since those extra impurities can disrupt downstream chemistry, especially in pharmaceutical R&D. Labels also mention batch numbers, shelf life—commonly two years in optimal storage—and reflect compliance with international standards, such as REACH or ISO. Laboratories track inventory with specifications including melting point, molecular weight, and safety pictograms that communicate hazards like skin corrosion and aquatic toxicity. Only certified technicians or trained researchers have access, since safety data sheets warn of risks on inhalation and skin contact. Documentation plays a central role, especially in regulated drug or agrochemical synthesis.
The typical industrial method relies on reacting catechol with chloroacetyl chloride in the presence of a base, such as pyridine or triethylamine, under cooled, controlled conditions. The exothermic nature of adding chloroacetyl chloride dictates the pace—steady drip feeds, vigorous stirring, and cooling baths are staples in pro labs. After the main reaction, extra base neutralizes acid byproducts, and multiple washes remove remnants. Vacuum distillation or recrystallization from ethanol or ether yields the final product. Waste management becomes critical at this stage, since any chloro compounds in runoff need decontamination before environmental release. Labs routinely review protocols to keep yields high and risks in check.
Few intermediates spark as much creative chemistry as 4-Chloroacetylcatechol. Its two free hydroxyl groups and the chloroacetyl side chain lend themselves to further transformation. Nucleophilic substitutions, especially with amines or thiols, break open new functional possibilities—forming new bonds for active pharmaceutical ingredients or customized polymers. Oxidation or methylation of the catechol core modifies reactivity and stability. Some research teams have shifted to greener solvents or milder conditions in these reactions, seeking both environmental gains and improved product consistency. Each modification opens new chemical avenues, especially in structure-activity studies for medicines targeting enzyme pockets or ion channels.
Over years, different labs and suppliers have published a long list of names for 4-Chloroacetylcatechol. The most common include 4-(Chloroacetyl)catechol, 2,3-Dihydroxyacetophenone-4-chloro, and CAS 6504-64-5. Some catalogs list the compound by regional names in German or Japanese textbooks, while others assign proprietary codes for tracking in inventory systems. These aliases grew out of historical naming conventions, with each reflecting either functional group arrangement or synthetic lineage. Chemists ordering from global suppliers depend on accurate cross-referencing to avoid mix-ups in high-impact applications.
Strict operational rules govern 4-Chloroacetylcatechol in labs and manufacturing plants. Direct contact causes irritation, and inhalation brings respiratory distress, so splash goggles, chemical gloves—usually nitrile or neoprene—and lab coats create a minimum barrier. Fume hoods run constantly when measuring or transferring powders, and chemical showers stand ready where production volumes exceed a few hundred grams. Chemical Hygiene Plans mandate safe disposal practices for both the compound and any contaminated gear. Emergency protocols detail quick responses in case of spillage, and most companies require regular audits, tracking incidents for continuous engineering improvements. Storage must isolate this compound from oxidizers or bases, as accidental mixing can lead to uncontrolled side reactions. Safety data, reviewed monthly, remind workers of the risks in every stage of handling.
Demand for 4-Chloroacetylcatechol runs high in focus areas like pharmaceutical synthesis, where it serves as a starting point for enzyme inhibitors, anti-inflammatory agents, or intermediates in complex natural product construction. In agricultural chemistry, designers use its reactive groups to prepare targeted herbicides and fungicides with improved environmental profiles compared with earlier generations. Polymer chemists exploit its aromatic core and side chains to build specialty resins and coatings that balance durability with tunable flexibility. A few electronics manufacturers experiment with this compound to modify polymer backbones for advanced films and conductive adhesives. Each industry has honed its protocols based on unique process needs, and small-scale contract synthesis teams often build custom analogs tailored to emerging market requests.
Active research projects push 4-Chloroacetylcatechol into new territory. One stream investigates further modifications to the catechol ring—adding halogens, alkyl, or nitro groups to probe changes in biological activity. Others focus on green chemistry techniques, seeking reduced solvent use, lower reaction temperatures, and renewable feedstocks. University groups pick apart the compound’s mechanistic roles in oxidative stress studies and pursuit of new anti-cancer leads. Patent filings point toward combination therapies attaching 4-Chloroacetylcatechol derivatives to peptides, targeting delivery to specific tissues. More basic research still focuses on structure-activity relationships, linking the position of the chloroacetyl group or substitutions on the ring to observable changes in catalysis or reactivity. Each breakthrough brings fresh insight into both synthetic applications and biological impacts.
Toxicity tests flag 4-Chloroacetylcatechol as a moderate hazard, particularly for eyes, respiratory systems, and aquatic organisms. Standard models in rodents show dose-dependent irritant and possible organ toxicity with repeated exposures, while aquatic toxicity screens emphasize restricted use near waterways. Chronic exposure risks prompt regular monitoring for occupational lung or skin sensitization. Regulatory agencies and safety committees review available data each year, often requesting more animal-free testing or better predictive modeling. The rising demand for green chemicals triggers a search for safer analogs or more effective personal protective equipment, especially as industrial volumes tick upward. Chemists tend to prefer containment, remote handling, and rapid neutralization protocols as safer approaches gain traction through industry partnerships.
The outlook for 4-Chloroacetylcatechol grows alongside new needs for high-performance materials and medicines. As reactive intermediates stay popular, research will likely keep uncovering novel applications—maybe in biodegradable plastics or targeted drug delivery platforms. Ongoing pressure from tightening safety and environmental regulation drives suppliers and formulators to develop purer, less toxic versions or enhanced containment strategies. AI-guided molecule design might speed up discovery of new derivatives, optimizing both reactivity and biocompatibility. As companies chase sustainability goals, production processes could pivot to bio-based catechols, and synthetic methods could see a step-change in safety or yield. This compound stands as both a challenge and an opportunity: responsible handling and innovative research unlock its full value across dozens of modern industries.
I’ve had my share of digging through dense chemical data to figure out what certain compounds really do out in the world. 4-Chloroacetylcatechol isn't a household name, yet it pops up in labs and research papers that sit behind dozens of paywalls. Even most chemists probably went years before running into it in any hands-on experiment. Curious folks overlook it because its uses sound so niche, but in reality, the compound threads through medicine, crop science, and even industrial research.
Pharmaceuticals often start life in labs full of bottles whose labels barely hint at their impact. 4-Chloroacetylcatechol usually steps in as a starting point, or what chemists call an “intermediate.” It helps build more complex molecules—antibiotics, anti-inflammatory agents, and even some drugs under development for neurological conditions. I’ve seen pharmaceutical chemists tinker with it to tweak properties of new compounds, aiming for better absorption or stronger activity against infections. That sort of research draws on the fact that small tweaks can mean new lifesaving medicines, which puts the compound in a crucial spot for healthcare innovation.
Nobody likes talking about chemicals on crops, but a lot of us enjoy a bug-free tomato. 4-Chloroacetylcatechol steps into some formulas that make pesticides more effective or less likely to drift into a neighbor’s field. Its chemical backbone lets scientists attach different side groups to generate new compounds, some of which target pests while sparing beneficial insects. My own experience reading agricultural reports taught me that one compound’s impact can ripple through the whole food chain, so every new ingredient faces plenty of hurdles. Local farmers in my area often ask if newer products could help them use less spray without sacrificing good harvests. Those kinds of improvements often come from small shifts in compounds in the pesticide world, and 4-Chloroacetylcatechol sometimes sits at the start of those innovations.
It would be a stretch to call 4-Chloroacetylcatechol a staple in material science, but advanced manufacturing firms have begun exploring its potential. Its unique chemistry opens doors to polymers and specialty materials. In my own time visiting manufacturing floors, I saw researchers keeping a close eye on cost, safety, and downstream waste. They probe molecules like this to tailor coatings, improve resins, or make adhesives for tricky applications—electronics for one, or medical devices that demand precision and purity. The research may not always make headlines, yet it lays key groundwork for safer and more resilient products.
No discussion about chemicals gets far without raising safety and access. Research in this area emphasizes rigorous safety testing. Exposure can irritate the skin and eyes, and laboratories must train staff on handling protocols. Governments and agencies put these safeguards in place after decades of learning from past mistakes. Experts recommend clear labeling and robust data sheets, making sure everyone—from university researchers to industrial chemists—reads up before opening a shipment.
4-Chloroacetylcatechol highlights how chemistry links science to solutions, but it also underscores the need for oversight and curiosity. Monitoring its use through peer-reviewed studies, transparency about results, and feedback from people closest to its effects all play a part. I always return to one guiding thought: chemicals create value, but trust grows from honest research and open conversation. The story of this compound reminds me that every innovation should match scientific rigor with public accountability—and that’s how we keep advancing responsibly.
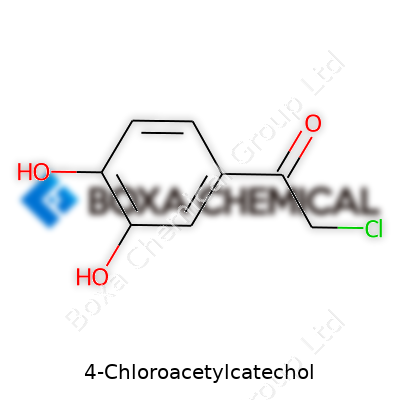
It’s easy to glance at a name like 4-Chloroacetylcatechol and brush it off as just another chemical in a textbook. Digging deeper, you find a molecular structure carrying its own unique fingerprint. Here’s what you get: two hydroxyl groups holding their places on a benzene ring, sitting at neighboring carbons to form catechol, then the real shift—an acetyl group touched by chlorine, attached at the fourth carbon. This structure reads like a map of connections, each change in position hinting at reactivity and purpose.
Chemists break down 4-Chloroacetylcatechol as C8H7ClO3. It starts with the classic catechol backbone—a benzene ring with hydroxyls at carbons 1 and 2—then adds a 4-chloroacetyl group at the para position. Draw it out and you see a clean layout: hydroxyl (–OH) groups are oriented for hydrogen bonding, the acetyl (–COCH2Cl) carries both carbonyl and halogen flavor, combining polar and slightly lipophilic features. A presence like this can shift biological activity, solubility, and reactivity in ways you only appreciate by working at the bench or reading papers that dig deep.
Structures like 4-Chloroacetylcatechol aren't just trivia for chemists. They show up in synthesis challenges, like building blocks for pharmaceuticals or starting points for polymers. Researchers need sharp eyes because a small swap—say, putting that chloroacetyl group one carbon away—results in a whole new molecule with its own properties. You see catechol derivatives featured in journals for their role in chelating metals, preventing corrosion, and adding activity to drugs. Substituents make all the difference: a chlorine bonds to the molecule and flips the script on how bacteria, enzymes, or solvents react to it.
It’s tempting to see chemicals just as neat structures, but the real story unfolds in labs, plants, and even outdoors. A halogen like chlorine raises red flags for toxicologists. Chlorinated compounds require respect: their presence in certain drugs can raise efficacy and lipophilicity, but in the wrong context, they can hang around in the environment or persist in living tissue. Catechol’s own reactivity makes it prone to oxidation, releasing quinones or even free radicals. Anyone handling, synthesizing, or discarding such compounds has to take personal safety and environmental protection seriously—lab coat, gloves, and a solid waste plan aren't optional.
No one should underestimate the impact of tiny chemical tweaks. A molecule like 4-Chloroacetylcatechol carries lessons for every chemist and student. Each functional group means something: hydroxyls interact with water and proteins, acetyl groups can open doors in synthetic pathways, chlorine atoms add reactivity and flag environmental questions. The exact structure impacts biological targets, manufacturing choices, and regulatory attention.
Experience in the lab shapes your view of chemicals like this. I've seen colleagues skip details, only to realize the structure dictated whether a reaction failed or a new property emerged. Companies and universities keep pushing for greener approaches, using better purification and safer solvents, by learning from how structures behave. Tools like computational chemistry and open-access papers help craftspeople and stakeholders predict which derivatives serve us best, while avoiding surprises in toxicity or persistence.
4-Chloroacetylcatechol belongs to a family of chemicals with some real baggage. Anyone who has stepped into a laboratory or chemical production floor knows the rulebook gets heavier when handling compounds like this. It enters through the skin, the eyes, the nose—and every route comes with risks. The compound’s structure flags not only irritation but also potential systemic toxicity, so tackling this stuff with bare hands or simple goggles is a bad idea.
Whenever I’ve worked with chlorinated aromatics, the first thing I check is personal protection. A good lab coat, made from a chemical-resistant material—not some worn cotton that soaks up spills—goes on before I even approach the bench. Gloves matter, and nitrile stands as the standard choice; latex can break down. After years of training, I stopped trusting myself to remember not to touch my face, so face shields and goggles became a non-negotiable habit. The stinging in my eyes from a lesser irritant long ago convinced me nothing beats eye protection.
Glove changes happen each session. Extended use, or worse—a tiny splash—calls for immediate disposal. I learned from a former colleague that gloves only delay exposure, so washing hands after every session turns into muscle memory. After one lab incident—no major harm done, but plenty of paperwork—I started inspecting gloves for microtears. Turns out, even small breaches open the path for irritation or real injury.
Good ventilation plays just as big a role as personal gear. Chemical fume hoods pulled my attention early on: proper airflow stops vapors from lingering at face level. Seeing a colleague struggle through a fume-induced headache once convinced me never to skip these setups, even for “quick” tasks. Never work with 4-Chloroacetylcatechol on an open bench—the risk just isn’t worth it.
Labeling and containment always show up in safety checklists. Containers, tightly sealed and clearly marked, take up shelf space at eye level—not on forgotten back shelves. Back in an internship, I found one bottle of a similar compound with faded writing and a brittle cap. After alerting the supervisor, we fixed labeling and upgraded storage across the lab. Mishaps with unknown or poorly contained chemicals almost always trace back to poor habits in organization.
Waste collection marks a dividing line between safe labs and accidents waiting to happen. I learned from an industrial chemist how regulatory obligations and environmental impacts demand attention. A hasty pour down a sink, aside from breaking rules, risks water contamination and fines. All 4-Chloroacetylcatechol waste goes in dedicated, labeled containers, sent for professional hazmat disposal. Training sessions taught us to double-bag any solids contaminated with the compound and to never mix incompatible waste streams.
No shortcut replaces a strong safety culture. Personal accountability, easy-to-read documentation, and emergency routines guide safe practice. I still print emergency numbers and keep them in arm’s reach, because flustered searching during an accident costs precious minutes. People sometimes believe accidents only happen to careless folks. My experience says the opposite: even experts slip up. Honest self-assessment and regular retraining keep everyone safer—this principle holds true for 4-Chloroacetylcatechol or any hazardous chemical along the bench.
4-Chloroacetylcatechol belongs to a class of chemicals that often triggers red flags for anyone handling specialty or research-grade materials. It doesn’t behave like table salt or sugars in your kitchen. This compound, with its reactive groups and chlorinated backbone, poses safety risks that call for structured storage rather than casual shelf space.
Setting up a safe storage environment for 4-Chloroacetylcatechol begins with basic chemistry awareness. The compound reacts when left near moisture or sources of heat—damage appears not only on the label but also in your results or, worse, on your skin. Reliability and accuracy in labs ride on avoiding these risks.
A dry, cool, and well-ventilated storage area always sets the stage for safety. Think of a simple habit: place any container with this chemical out of direct sunlight. Anyone who’s rushed a reaction under bright lab lights remembers how fast things can go wrong. High temperatures don’t just spoil your sample, they risk dangerous reactions or leaks.
A tight-sealing glass or high-quality plastic container works best. The container shouldn’t show even a hint of wear. Having handled various reagents over years in research labs, a stubborn lid or chipped jar always made me uneasy. Damaged lids or weak containers invite moisture and contamination, sending your reagent straight to the waste bin. Add clear labeling with the name, date, and hazard warnings right on the vessel. If a bottle ever arrives in a secondary container, keep it that way. Spill containment trays have rescued more than a few benches from messes nobody enjoys cleaning.
Many chemicals require neighborly consideration, but 4-Chloroacetylcatechol appreciates solitude. Place it away from oxidizers, acids, or bases. Real-world experience shows that even smells can travel, so a designated chemical storage cabinet offers real peace of mind. Locked storage, out of reach from casual hands, reduces exposure for everyone, especially in university or shared laboratory spaces.
Training and signage play as critical a role as the container itself. Making safe storage part of onboarding for anyone joining a lab can prevent those moments of uncertainty that often lead to mistakes. I still remember the feeling after a colleague reached for the wrong bottle because the shelf lacked labels—a simple fix that keeps everyone safer.
Spill kits sit right beside the storage area in responsible labs, and they aren’t just there for show. Accidents happen quickly, often during rushed experiments. Quick access to protective gear such as gloves, goggles, and fume hoods protects against short- and long-term health problems, and I’ve seen how forgotten gloves lead to avoidable clinic visits.
Inventory checks and regular cleaning not only prevent clutter, they serve as reminders to check container integrity and confirm nothing hazardous is overlooked. Every thorough inventory doubles down on safety, highlighting leaks or expiration before they become disasters.
Storing 4-Chloroacetylcatechol isn’t just about following codes—it’s about protecting people and the science they carry out. Consistent habits, accessible safety tools, and a little vigilance every day build a trustworthy lab environment.
Chemistry can feel distant until you dig into what the numbers mean for real-world work and research. The molecular weight of 4-Chloroacetylcatechol, which comes out to 200.59 g/mol, might look like just another data point in a chemical catalog. For researchers and formulators, that single figure packs practical punch. Molecular weight drives everything from dosing and mixing to reaction scaling.
Back in college, I ran titrations with catechol derivatives. It didn’t take long to see that a mistake in molecular weight would send an entire calculation off the rails. Mixing up a few tenths of a gram could shift a reaction’s balance, drain a budget, or destroy an experiment’s day. Precision means fewer headaches and, importantly, keeps the science solid.
Let’s say a chemist wants to prepare a solution of 4-Chloroacetylcatechol at a certain molarity. Each milligram connects directly to molecular weight. Getting that figure right determines whether the solution helps or hinders. Even the environmental impact of waste or the risk assessment for handling hazardous materials comes back to accurate weights.
For anyone in pharmaceuticals, fine chemicals, or materials, the molecular weight isn’t just trivia. Scale-up teams calculate solvent volumes, reactor charges, and process yields off that number. In pharmaceutical synthesis, for example, dosing pivots on milligram precision. Regulatory filings for new drugs demand exact calculations because each error can ripple into safety, effectiveness, or compliance.
Analytical chemists lean on molecular weight for mass spectrometry, chromatography, and purity checks. It shapes spectral peaks and confirms compound identity. When teams troubleshoot a reaction or scale up a batch, they want confidence those peaks belong to a single, well-characterized compound.
Errors in molecular weight calculation do real harm. Labs working fast sometimes grab numbers off internet tables without double-checking the chemical structure. When a new chemist calculated the molecular weight for a chloroacetyl derivative by copying numbers for a similar catechol, the results wasted hours and expensive materials. Instead, it helps to pull up the structure, connect the atomic weights—chlorine, acetyl, catechol core—and confirm each step. That tiny step of double-checking brings peace of mind, not just to the bench scientist, but down the entire production and regulatory stream.
In my own process troubleshooting gigs, just tracking down a molecular weight error often corrected months of inconsistencies in batch records. Most chemists start assignments with a quick exercise: draw the structure, count each atom, add up the weights, and look for outliers. That habit saves reputation and money.
Strong workplace habits keep mistakes down. Training teams to confirm molecular weights as part of their SOPs works well. Building culture around double-checking not only catches errors, but also encourages young chemists to ask questions and become experts in their own right.
Modern tools, like chemical drawing software and curated online databases, give a second way to verify each entry. As the industry sees more automation and digitalization, these checks stay important. Molecular weight may sound simple, but treating it with respect pays dividends up and down the pipeline.
| Names | |
| Other names |
2,3-Dihydroxyphenyl chloroacetate Chloroacetylcatechol |
| Pronunciation | /ˌfɔːr-klɔːr.oʊ-əˈsiː.tɪl-ˈkæt.ə.kɒl/ |
| Identifiers | |
| CAS Number | [481-86-7] |
| 3D model (JSmol) | ``` JSmol.loadInline('data/mol:CC(=O)ClC1=CC(=C(C=C1)O)O') ``` |
| Beilstein Reference | 1462041 |
| ChEBI | CHEBI:84582 |
| ChEMBL | CHEMBL203911 |
| ChemSpider | 177046 |
| DrugBank | DB08226 |
| ECHA InfoCard | ECHA InfoCard: 100.010.376 |
| EC Number | 4.1.1.1 |
| Gmelin Reference | 60486 |
| KEGG | C12345 |
| MeSH | D048382 |
| PubChem CID | 166145 |
| RTECS number | GG0875000 |
| UNII | S0633T35RB |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C8H7ClO3 |
| Molar mass | 185.59 g/mol |
| Appearance | White to yellow crystalline solid |
| Odor | Odorless |
| Density | 1.439 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.78 |
| Vapor pressure | 4.2 x 10^-4 mmHg (at 25°C) |
| Acidity (pKa) | 11.5 |
| Basicity (pKb) | 13.64 |
| Magnetic susceptibility (χ) | -52.0e-6 cm³/mol |
| Refractive index (nD) | 1.6490 |
| Viscosity | Viscosity: 1.63 mPa·s (at 25 °C) |
| Dipole moment | 2.86 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 344.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -322.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1427.9 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS05 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H332, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P304+P340, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | 122 °C |
| Lethal dose or concentration | LD50 oral rat 283 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Chloroacetylcatechol: "199mg/kg (rat, oral) |
| NIOSH | GR0525000 |
| PEL (Permissible) | No established PEL |
| REL (Recommended) | 300 mg/L |
| IDLH (Immediate danger) | Not established |