4-Bromocatechol has a backstory that feels familiar to many aromatic compounds known in organic chemistry. Researchers looking to tweak aromatic rings noticed that adding halogens like bromine made a big difference in reactivity and application. By the mid-20th century, 4-bromocatechol took on a specific role, often linked to dye synthesis and as an anchor point for building more complex molecules. Lab books from older university research centers often mention its preparation when seeking a functionalized catechol for cross-coupling experiments or when searching for effective intermediates in medicinal chemistry. The journey from basic catechol to brominated derivatives highlights how curiosity in tinkering with phenolic chemistry has led to a collection of tools valuable for both academia and industry.
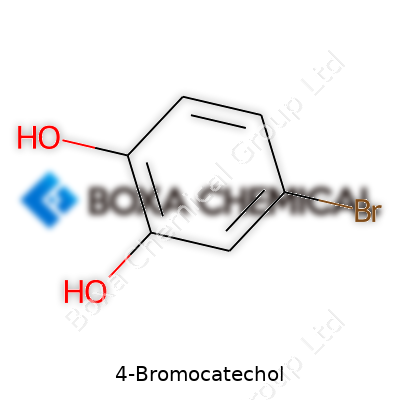
4-Bromocatechol stands as a solid chemical choice for scientists who want functionality and flexibility from an aromatic building block. Its chemical formula, C6H5BrO2, gives it a recognizable structure: a benzene ring featuring two hydroxyl groups placed ortho to each other with a bromine at the para position relative to a hydroxyl. Companies and labs treat it as a specialty raw material, valued for use in small-scale syntheses and custom compound production rather than massive commercial batches. Chemists know it for reliable performance in reactions demanding electron-donating groups positioned ortho to a halogen, which can control downstream reaction selectivity.
This compound offers a faint off-white to pale beige crystalline powder, depending on its purity and method of drying. Melting points often center right around 120–124°C. It dissolves slightly in cold water, better in hot water, but shows much greater solubility in organic solvents such as ethanol, ether, and chloroform. Its odor tends toward mild phenolic, without the pungency some halogenated benzenes carry. As with many brominated catechols, this molecule stands out due to its dual reactivity: the hydroxyls can chelate metals or engage in hydrogen bonding, and the bromine paves the way for substitution or metallation. This combination sets it apart in both bench chemistry and applied research.
Most suppliers and standards organizations advise clear labeling that reflects not just the compound's identity but its batch purity, physical state, and relevant hazard warnings. The material often ships in double-sealed, amber glass containers that keep light and moisture at bay. Typical purity ranges start above 97%, though analytical research demands material testing above 99%. Labels indicate the CAS number 5111-18-2, molecular weight at 189.01 g/mol, and storage recommendations: cool, dry, away from incompatible compounds like strong oxidizers. Any deviations from technical benchmarks—such as melting point or color—should get flagged for review before research use.
There’s more than one way to make 4-bromocatechol, but most routes involve either direct bromination of catechol or selective demethylation of 4-bromo-1,2-dimethoxybenzene. The direct bromination route uses elemental bromine and careful temperature management to keep reaction control tight and prevent over-bromination, with water or glacial acetic acid as the typical solvent. After reaction completion, cooling the mixture precipitates crude product, then repeated recrystallization from ethanol or water purifies it. Alternative methods—particularly for higher purity or scalability—opt for methyl-protected intermediates, adding a bromine atom on the protected ring, then demethylating to yield free catechol groups. Chemists weigh yield efficiency against operational safety, favoring low-chlorinated equipment and good ventilation, since halogenations release corrosive vapors.
Anyone working with 4-bromocatechol quickly finds that its true strength comes through its versatility. The phenolic hydroxyls open doors for etherification, acylation, and oxidative coupling, making it a go-to substrate for the synthesis of polymers, dyes, and complex ligands. The bromine atom supports Suzuki and Stille cross-coupling reactions, which allow scientists to link up aromatic groups under mild, palladium-catalyzed conditions. This site-specific reactivity helps forge new biaryl systems, and, in a pharmaceutical context, gives medicinal chemists a foundation for introducing functional groups that tune biological activity. Laboratory experience shows that ring bromination at the para-position rarely interferes with ortho-based substitutions, so the compound acts as a flexible intermediate for multi-step synthesis projects.
4-Bromocatechol circulates in literature and catalogs under more than one name. Some refer to it as 4-bromo-1,2-benzenediol, while older references call it 1,2-dihydroxy-4-bromobenzene. Shortened nicknames come up too, such as "Bromo-Catechol" or "Para-Bromocatechol." Chemical suppliers sometimes expand lists to include other languages or trade-related codes but stick to CAS 5111-18-2 for certainty.
Handling 4-bromocatechol does not rival the hazards of strong acids or reactive metals, but it comes with its own risks. Operators wear gloves and goggles, not just as a formality, but because skin contact can cause irritation and inhaling the dust irritates mucous membranes. Spills demand attention right away, preferably with absorbents that prevent spread and chemical burns. Most research labs rely on fume hoods for operations that involve heating or reactions with acids or bases. Disposal plans for waste follow local regulations and never pour it down the drain; phenolic waste, especially those containing halogens, requires incineration at certified facilities.
This chemical found a home in materials development and synthetic work, most notably as an intermediate for pharmaceuticals and agrochemicals. Dye manufacturers prize it as a precursor for specialty azo and quinone dyes where precise substitution patterns matter. Analytical chemists use it to create ligand frameworks for spectrometric and chromatographic standards. In certain environmental research, scientists employ 4-bromocatechol as a marker to track degradation pathways or enzymatic transformation of halogenated aromatics. The molecule’s combination of hydrophilic (hydroxyl) and hydrophobic (bromine) features lets it slip into diverse projects, from small-format research to exploratory product development.
Brominated catechols like this attract continued attention from both industrial R&D and academic researchers. Labs focus on improving methods for greener synthesis and recycling, reducing hazardous reagents, and replacing volatile solvents with more sustainable choices. Novel polymer systems and smart ligands often begin with a 4-bromocatechol scaffold, as customization possibilities prevent stagnation in product streams. The fine chemical sector continually probes new applications in dye and pigment manufacturing or seeks medical candidates in early-stage drug screening. In academic circles, the compound helps students grasp central concepts in aromatic substitution, cross-coupling, and phenol chemistry.
Published animal studies indicate moderate toxicity; high doses can depress the central nervous system, as seen in related catechols. Skin and eye contact sometimes bring strong irritation, and long-term exposure—especially in conditions with poor ventilation—could result in sensitization reactions. Environmental researchers continue measuring its breakdown rates in water and soil. They focus closely on byproducts: incomplete biodegradation might lead to compounds considered more toxic or persistent. Regulatory agencies keep an eye not just on immediate toxicity, but also on the cumulative effects on ecosystems, paying close attention to laboratory and industrial wastewater controls. Users need real-world data, not just theoretical LD50 values, to set exposure standards and develop practical protocols for containment and cleanup.
Looking ahead, 4-bromocatechol appears ready to take on greater roles in both specialty chemistry and green engineering. As chemical companies look to fine-tune halogen atom use in organic synthesis, brominated catechols could provide templates for biodegradable pesticides or more selective pharmaceuticals. In the realm of catalysis and sensor development, attaching flexible functionalities to the aromatic ring supports innovation. The shift toward sustainable production methods will place more focus on greener synthesis, alternative solvents, and renewable feedstocks, but 4-bromocatechol stands out as a bridge between traditional organic chemistry and tomorrow's more selective, responsible production models. Research already leans on it as a benchmark for reactivity and functional diversity, underscoring its value both in today's labs and tomorrow's manufacturing floor.
4-Bromocatechol gives a good example of how a small change in a molecule alters properties and potential uses. The molecule builds on catechol, which itself is best known for having two hydroxyl groups side by side on a benzene ring. In this variation, the 4-position on the benzene ring gains a bromine atom. Chemically, its formula reads C6H5BrO2. To picture it, the benzene ring presents as a hexagon, with -OH groups at carbons 1 and 2, and a -Br at carbon 4. Plain catechol has a sweetish chemical smell, and the addition of bromine can bump up both its reactivity and its possibilities in research and industry.
In the world of molecules, adding a halogen like bromine to a basic scaffold such as catechol shifts not just its smell but its whole reactivity pattern. That one atom makes a difference in how the substance interacts with living systems—or with other chemicals in a lab. I’ve run syntheses where a brominated building block brings entirely new reactivity. Often, a substituted catechol like this can serve as a stepping stone for making more complex organic compounds. Pharmaceutical labs see value in these tweaks because bromine can act as a placeholder; you can swap it out for another group later or use it to direct where other reactions happen.
In practice, chemists use 4-bromocatechol to build other molecules you’d never guess connect back to it. Sometimes it turns up in the early stages of making dyes or pharmaceuticals. Researchers also turn to it when embarking on the development of new ligands for catalysis. The bromine changes how enzymes or catalysts recognize the molecule, and that can help test theories about molecular recognition or toxicity.
To me, the most interesting part comes from its electron-donating and electron-withdrawing features. The hydroxyl groups often lend electron density, making the ring more reactive, while the bromine tugs the electrons in the opposite direction. That push-pull in the structure acts a bit like tuning a guitar—shift a string, and the whole resonance changes. Those who work in chemical synthesis pay attention to such details because it means the difference between a clean reaction and a roomful of unwanted byproducts.
No commentary is complete without addressing challenges. While 4-bromocatechol opens doors for creative chemistry, it carries risks. Brominated compounds frequently resist degradation, and exposure can raise health and environmental flags. I’ve seen colleagues double check waste labeling and protective gear when using such molecules in the lab, knowing that skin contact or inhalation could lead to irritation or worse effects. These days, smart chemists try to design reactions that limit side-products or use greener alternatives where possible. Still, certain syntheses need what only a molecule like 4-bromocatechol can offer, especially in specialty pharmaceutical or material science work.
Insight from basic organic chemistry—like tracing the subtle differences between catechol and its brominated cousin—pays off in both academic research and product innovation. Whoever wants to push the limits of medicine, electronics, or environmental cleanup often starts by deeply understanding the properties and risks tied to these molecular shifts. Open discussions, detailed documentation, and respect for both benefit and hazard keep progress safe and meaningful.
4-Bromocatechol stands out to chemists focused on organic synthesis and material science. This compound carries a bromine atom tucked into its catechol structure, making it more reactive. Its reactivity draws interest, especially for labs hunting for new building blocks to speed up research in pharmaceuticals, agricultural chemicals, and materials.
Consider the early phase of drug development. Medicinal chemists often want to tweak molecules by plugging in different functional groups. The bromine atom on 4-Bromocatechol gives them that entry point. Through a reaction called cross-coupling, chemists can bolt on a wide variety of groups to the catechol core. This process sets the stage for creating test drugs when traditional catechol isn’t quite reactive enough.
I’ve talked with a few researchers who say brominated catechols have opened new doors, especially when working with enzyme-inhibiting drugs or anti-cancer candidates. Their unique arrangement helps explore more chemical space, which is critical in a field where every slight tweak to a molecule can affect safety or effectiveness.
Material scientists have a different target. They’re looking for compounds that can form strong bonds to metals and create coatings or films. Catechols stick to metal like glue—think mussels holding tight to rocks—and the bromine atom adds a new reactive handle. Researchers can transform this handle into something else. This adaptability gets used to make corrosion-resistant coatings, sensors, or adhesives that perform under harsh conditions.
Some of the big names in electronics and advanced manufacturing experiment with catechol derivatives to build conductive polymers or enhance bonding in composite materials. The modification with bromine means more flexibility during manufacturing. That’s important in industry, where changing a process can stall a production line unless you have compounds that work smoothly from the start.
Lab chemists need versatile intermediates. 4-Bromocatechol’s structure lets it serve as a branch point to synthesize more complex molecules. They use it to make agrochemical candidates, antioxidants, and specialty dyes. Its structure offers entry to further bromination, oxidation, or elaborate coupling reactions, which are the backbone of modern synthetic chemistry.
Many of these routes took off because the bromine gives a specific location to modify the catechol ring. Without that anchor, some modifications would sprawl, causing unwanted by-products or lowering yields. My experience in small molecule synthesis taught me that this kind of selectivity saves time and resources, especially when juggling deadlines and budgets in the lab.
Any discussion needs to cover practicality. Like other halogenated aromatics, 4-Bromocatechol isn’t the friendliest chemical on the shelf—direct exposure can irritate skin and eyes, and inhaling dust or vapor raises safety concerns. Most labs train staff to use gloves, goggles, proper ventilation, and to store the compound sealed tightly. OSHA and EPA guidelines stress safe disposal and spill response, so the benefit of innovation goes hand in hand with respect for safety.
The chemical industry’s track record isn’t spotless, and ongoing research looks for cleaner, safer alternatives. Some universities and startups explore greener synthetic chemistry routes using renewable resources or milder reagents to make these building blocks. Others invest in automation that reduces handling risk. This new wave could balance progress with environmental and health standards, making the benefits of 4-Bromocatechol more widely accessible—without the baggage of outdated hazards.
I’ve worked in chemical research labs where every bottle on the shelf tells its own story. 4-Bromocatechol stands out as a compound that demands respect. As an aromatic brominated catechol, this powder has properties that can cause issues if you get careless. Safety isn’t about ticking off items on a list; it means treating substances with a level of caution that reflects your own experience, your team’s safety, and the track record of accidents that shaped proper lab protocols.
In my experience, the temptation to “just quickly measure out the powder” catches up fast. Good gloves—either nitrile or neoprene—stop skin absorption and block the risk of sharp allergens. Always pick fresh gloves for each session because microtears build up fast, sometimes without anyone noticing. Safety goggles protect against fine dust, which escapes easily and irritates eyes painfully. I once saw someone shrug off a pair of goggles because they’d “never had a problem before.” About half an hour later, a tiny puff from the bottle led to a red, swollen eye for days. It’s never worth it.
Fine powders like 4-Bromocatechol drift everywhere. Standard dust masks don’t cut it here. At my last job, the lab stocked up on fitted respirators with cartridges certified for organic vapors and particulates. Even with great ventilation, using a fume hood isn’t optional. Airflow in a good hood removes escaped dust, keeping exposure low. Always check for enough draw by holding a small paper strip near the opening—if it doesn’t pull inward, airflow isn’t strong enough. Relying on an old or broken fan gets people in trouble more often than not.
Small spills used to send folks scrambling for paper towels. These days, chemically resistant mats and spill absorbent kits stand ready. If powder lands on skin, head straight to the sink. Wash with lots of water. Scrubbing spreads it deeper. For larger spills, a dedicated protocol takes over: cordon off the area, clean up with proper gear, and let the safety team double-check. Industry stats show that early, calm cleanup means the difference between minor incidents and medical bills.
Storing 4-Bromocatechol somewhere dry means avoiding both moisture and sunlight. Humid air degrades catechols and sometimes creates sticky, toxic messes. My old supervisor always labeled the bulk powder with the opening date—if it sat longer than six months, we’d replace it. Sealed glass bottles with tight-fitting stoppers work best, and never share tools between reactive substances. It’s easy to brush off storage as “housekeeping” until someone grabs a mystery jar and ends up in trouble.
Labs rack up chemical waste fast. For brominated catechols, never dump leftovers in the sink. Instead, collect waste in labeled containers and call for specialist pickup. In 2019, improper waste disposal landed a university with a six-figure fine. Responsible disposal reflects trust in your workplace and the broader community; it’s not just about rules—it’s about pride in a job done right.
No matter how many chemicals I’ve handled, I always check the safety data sheet before working with a new batch. Refresher training on hazard communication and spill drills mean the next new hire stays as safe as the old-timers. Clear communication, a willingness to raise concerns, and a culture where safety gets constant attention save more lives than the fanciest gear.
Stepping into a lab, you can’t ignore how careful everyone gets with numbers. One miscalculation, and your results throw everything off. That’s what I think about each time I weigh out something like 4-Bromocatechol. The molecular weight of this compound sits at 191.01 g/mol. This number comes from the combined atomic masses of four carbons, five hydrogens, two oxygens, and a single bromine atom. Without proper numbers, entire experiments risk falling apart.
Chemical synthesis lives and dies by a balanced equation. For reactions involving 4-Bromocatechol, mixing the correct molar ratios sets the stage for reproducible work. I remember running into trouble in college, using outdated reference tables and getting a different number than the actual 191.01 g/mol. The product yield dropped, and the analysis didn’t add up. Mistakes like that slow research and eat up resources. Precision turns into a habit, and using the right molecular weight forms the backbone of reliable science.
Bromine-containing chemicals demand respect. Too much or too little can either waste expensive reagents or cause unexpected reactions that endanger the whole workspace. Dosing starts with the correct molecular weight. I’ve seen safety incidents born from rushed calculations, and once you smell burnt plastic in the fume hood, you never forget. Tight control over quantities, right down to the last decimal, moves lab work from risky to smart. Knowing each component well enough takes away some of the hazard and confusion.
4-Bromocatechol finds uses in pharmaceutical intermediates and synthetic dyes. The world outside the lab depends on exact formulation. Imagine a pharmaceutical company rolling out a medication with too much or too little of an ingredient. Nobody trusts a process that can’t match its numbers. Industry partners keep checking these calculations because a batch sent off-spec translates into costly recalls or rejection. Lives hang on these details, not just pride in benchwork.
People outside of chemistry sometimes wonder why we fuss over these numbers so much. There’s a culture in science built on transparency. Anyone should get the same answer if they repeat someone else’s experiment—and that’s only possible with standardized data. To build that trust, I log every step, include reliable data sources such as official chemical registries, and double-check with updated material safety data sheets.
Everyone who spends enough time around chemicals comes up with a few tricks. I keep digital reference tables of molecular weights from sources like the PubChem database. Typo-proofing through digital calculators and peer-verification makes a difference. I also mark hazardous compounds and double down on calculations before measuring solids like 4-Bromocatechol in the open lab. Mistakes get noticed fast, so learning how to ask for a second set of eyes on any calculation isn’t weakness; it’s wisdom. Lab culture rewards those who slow down enough to do arithmetic right the first time.
4-Bromocatechol sounds like something meant for a high-security lab, but it can show up in many research and industrial routines. This compound looks like an off-white solid, sometimes brownish if it’s out for a while. From my chemistry days in grad school, even a little moisture in the air could nudge such compounds to break down or release fumes, so those working with it carry a bit of respect for the bottle on the shelf.
This chemical isn’t just another dusty powder—left out, it can attract moisture and start to decompose. Once, a colleague forgot to seal up a jar and came back to a sticky mess that nobody wanted to touch. Not only does exposure make a mess, but it can also produce by-products that raise health risks. For people handling it: skin, eyes, and lungs aren’t safe without proper barriers. Always glance at the safety data sheet before popping open that container.
A regular cabinet won’t cut it. 4-Bromocatechol belongs in a tightly sealed glass or high-quality plastic container, away from sun or bright lab lights. Think of storing it like keeping a carton of milk fresh—cold, dry, dark. Most labs set aside special shelves that stay under 25 degrees Celsius. Ever since I started research, it’s become a kind of ritual to double-check the lids and labels every week—errors cost both time and health.
Desiccators aren’t old-fashioned in this context. Many labs keep desiccators filled with drying agents like silica gel. Tossing the container in here after each use blocks out the humidity completely. If you see even a hint of clumping, it’s time to switch to a fresh stash and clean up the remains. A small investment in a reliable desiccator pays off in peace of mind.
Anyone who has dealt with a hazardous chemical spill appreciates the need for planning. In my experience, supplies like nitrile gloves, splash-proof goggles, and fume hoods should be out and ready before opening the jar. Regular training keeps both newcomers and old pros alert. Every spill drill, every review of the material’s hazards—these habits aren’t just red tape, but the backbone of safety.
Labeling never goes out of style. Clear, bold text listing the chemical name, hazard symbols, and storage protocol staves off accidental mix-ups. If the label starts to smudge, swap it out right away. Unmarked containers feed confusion, and mistakes in a chemical lab rarely go quietly.
More labs are waking up to the realities of modern safety expectations. Digitized inventories help track batch dates and flag old stock before it spoils. Improved ventilation systems pull away any unexpected fumes from loose containers. Investing in these upgrades comes from hard lessons learned, and offers both protection and lower chemical waste.
The cost of proper storage always falls short of the potential fallout from one moment of carelessness. Careful handling and clear protocols turn risky materials into manageable tools. Every time someone picks up that jar of 4-Bromocatechol, the steps taken to keep it stable and secure pay dividends in safety for everyone nearby.
| Names | |
| Preferred IUPAC name | 4-Bromo-1,2-benzenediol |
| Other names |
4-Bromo-1,2-benzenediol 1,2-Dihydroxy-4-bromobenzene 4-Bromobenzene-1,2-diol |
| Pronunciation | /ˌfɔːrˌbroʊmoʊˈkætɪˌkɒl/ |
| Identifiers | |
| CAS Number | 53314-21-9 |
| Beilstein Reference | 1207783 |
| ChEBI | CHEBI:51375 |
| ChEMBL | CHEMBL153338 |
| ChemSpider | 66145 |
| DrugBank | DB08336 |
| ECHA InfoCard | 100.009.075 |
| EC Number | 4.2.1.22 |
| Gmelin Reference | 57218 |
| KEGG | C06587 |
| MeSH | D017957 |
| PubChem CID | 13717 |
| RTECS number | GF5950000 |
| UNII | 3P7A3G6A99 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C6H5BrO2 |
| Molar mass | 207.01 g/mol |
| Appearance | White to beige crystalline powder |
| Odor | Odorless |
| Density | 1.9 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.9 |
| Vapor pressure | 1.72E-4 mmHg at 25°C |
| Acidity (pKa) | 9.2 |
| Basicity (pKb) | 9.37 |
| Magnetic susceptibility (χ) | -72.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.680 |
| Viscosity | 1.740 cP (50°C) |
| Dipole moment | 1.6305 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 110.6 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | –39.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3680.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0-W |
| Flash point | Flash point: 138.3°C |
| Lethal dose or concentration | LD50 (oral, rat): 175 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Bromocatechol: **2400 mg/kg (rat, oral)** |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/L |
| Related compounds | |
| Related compounds |
Catechol 4-Chlorocatechol 4-Bromoanisole 4-Bromophenol |