Chemistry rarely moves in straight lines. Ideas and needs shape the creation and use of substances like 4-Amino-m-Cresol, or 3-methyl-4-aminophenol. Its discovery emerged alongside advances in phenolic chemistry during the late 19th and early 20th centuries, a period packed with innovation in dyes and pharmaceuticals. Laboratories chasing deeper colorfastness or new antiseptics stumbled upon phenol derivatives with new potential. Early work with toluidines, cresols, and their amino derivatives gave rise to dozens of new intermediates. 4-Amino-m-Cresol has since carved out a place, not through discovery fanfare, but through practical need and the incremental way science unfolds.
This compound belongs to the group of aminophenols, structurally marked by a methyl group and an amino group hanging on a benzene ring. It crosses the boundaries between academic research and large-scale industry, thanks to characteristics that fit into several niches. For years, dye manufacturers and hair color developers have used it for its powerful coloring properties, finding consistency and stability in its reactions. Chemical suppliers recognize it by several trade names and labels, often adjusting the purity or handling instructions for their audience, from research chemists to factory workers.
4-Amino-m-Cresol usually appears as a pale yellow to brownish crystalline solid. It will dissolve in water only sparingly but more readily in alcohols and other organic solvents like ether or chloroform. Its melting point hovers around 125–128°C, while its molecular weight sits at 123.15 g/mol. The chemical structure, C7H9NO, keeps reactions versatile but not too reactive under ambient storage. Fresh batches keep well in tightly sealed containers, away from direct light and air that could swing its performance.
Technical sheets spell out purity—usually 98% and above for industrial or laboratory use—with levels of related impurities and byproducts. Color index numbers, batch traceability, and lot numbers stay listed for ease of regulation and recall needs. Labels feature GHS hazard warnings, signal words, risk and safety codes, proper storage advice, and specific directions for accidental contact, tailored for anyone from warehouse staff to lab technicians. Documentation supports every shipment, as national and international regulations expect transparency.
Synthesis of 4-Amino-m-Cresol follows classic aromatic chemistry routes. Most often, chemists start from m-cresol and carry out nitration under controlled temperature, followed by reduction—frequently with iron filings and hydrochloric acid. Each step demands close monitoring: nitration can run fast and hot, while reduction risks partial conversion without careful heat and reagent addition. Recrystallization purifies the product, often from ethanol or water, rounding out a straightforward yet detail-driven process.
This aminophenol stands as a versatile building block for other reactions. In dye manufacturing, the amino group reacts with aromatic diazonium salts for azo coupling, delivering lasting color in textiles or cosmetics. Modification specialists exploit methyl and amino positions to create custom molecules for research or industry. Its reactivity opens doors to hundreds of derivatizations, including acylation or alkylation to match different performance targets. Over decades, the pharmaceutical world has explored this flexibility for drug intermediates, though most applications circle back to coloration.
Naming can get muddled across catalogs or geographies. Look up 4-Amino-m-Cresol and see titles like 4-Amino-3-Methylphenol, 2-Hydroxy-5-methylaniline, or p-Aminom-cresol. CAS numbers remove guesswork, usually tying companies, labs, and regulators to a common definition. Trade names sometimes disguise the chemical a bit for commercial advantage, but technical sheets confirm the real identity. In the dye trade, color index references or shorthand codes also make frequent appearances.
Handling 4-Amino-m-Cresol comes with the full slate of chemical hygiene protocols. Direct skin or eye contact has brought about irritation and allergic responses in workers, so personal protective gear goes beyond gloves and goggles to include full coverage in ventilation-controlled environments. Spills demand swift attention, using absorbent materials and closed disposal. Safety data sheets line out threshold limit values, disposal pathways under RCRA and national hazardous waste codes, and emergency response procedures, ensuring compliance at each handling stage. Regulators in Europe, the United States, and other jurisdictions have kept it under watch, especially since consumer products reach millions of homes every year.
Most of this compound goes into the dye and pigment sector, especially as an intermediate for oxidative hair dyes. Its coloring power stems from easily forming intense, stable phenazine or indamine structures on hair fibers—effectiveness that built trust among cosmetic chemists and multitonal dye product developers. Beyond cosmetics, 4-Amino-m-Cresol finds a role in making specialty pigments, some pharmaceutical intermediates, as well as research reagents in organic labs. Regulatory shifts over time, especially for products near food or skin, have nudged companies toward careful risk management or alternative ingredients, but demand for this established molecule remains robust.
Chemists and consumer health groups continue probing the boundaries of 4-Amino-m-Cresol. For coloring, R&D centers push for new shades and safer, less allergenic formulations without losing vibrancy or shelf-life. Analytical chemists refine purity specifications and new detection methods for trace contaminants, aiming to catch every last percentile of unwanted side products. Toxicologists support their case with improved biocompatibility tests and long-term exposure analysis. Green chemistry pushes for sustainable synthesis, examining everything from eco-friendlier catalysts to solvent swaps, reflecting growing environmental expectations.
Debate continues about the chronic and acute health effects tied to 4-Amino-m-Cresol. Short-term exposures often lead to skin and eye irritation; larger doses have produced toxicity in animal studies, with organ-level impacts and mutagenicity under certain test conditions. Long-term studies on humans remain sparse, though risks amplify for those handling the pure compound repeatedly. Global agencies, including the European Chemicals Agency and FDA, sift through these data when reevaluating ingredient lists for consumer goods. Scientists keep a close watch for any links to carcinogenicity, allergy buildup, or environmental persistence, motivated by both worker health and public safety.
Markets rarely stand still, and the future of 4-Amino-m-Cresol will depend on innovation, transparency, and tighter regulation. Cosmetic companies invest in research partnerships to create hybrid or biocompatible coloring agents, though substitutes usually struggle to match legacy performance. Regulations may restrict specific applications if new data show greater hazards, forcing shifts to alternative chemistries. On the technology front, green routes to traditional dyes could reduce the environmental burden, providing a sustainability pathway for decades-old molecules. Science and consumer trust balance each other, making ongoing research and open safety data key to responsible use ahead.
4-Amino-m-cresol sounds pretty technical, but it plays a role in places you might not expect, especially if you’ve ever dyed your hair. Sometimes listed on cosmetic labels, this compound helps shape the results of oxidative hair coloring products. It’s not the most famous ingredient, but it brings color science to the bathroom mirror. Chemical processing lifts the original color, then other molecules work together to build the new shade. 4-Amino-m-cresol reacts within these complex formulas, offering specific hues and providing long-lasting results. It isn’t the main dye, but helps fine-tune the color. The chemical structure lets it blend with other agents to craft subtle, natural-looking tones instead of flat, single-note results.
Regulators like the European Chemicals Agency and the U.S. Food and Drug Administration keep a close eye on compounds used in personal care products. Companies test for safety and list ingredients for full transparency. Public health depends on this ongoing effort. When I worked behind the counter at a salon, clients always asked about hair dye safety. Many had allergies or sensitive skin, so knowing what ingredients do helped us keep them safe. 4-Amino-m-cresol does have a risk of allergies in rare cases, so reading the packaging and doing patch tests remains important. Even a tiny amount can irritate sensitive people.
Hair dye gets all the headlines, but the uses for 4-Amino-m-cresol spread into other areas too. Lab researchers turn to this compound for organic synthesis. It acts as a building block for specialty dyes, pigments, and other aromatics found in textiles and plastics. One path leads to photographic developers, especially for older film technologies. Chemists value its structure for creating molecules that can change how materials absorb or reflect light. There’s a web of specialty manufacturing—tools, coatings, art supplies—where color consistency matters. Each batch of pigment or plastic might include small but critical doses of ingredients like this, keeping colors bold and true over time.
Like most industrial chemicals, responsible handling is essential. Research shows that excessive exposure can hurt skin or eyes, and inhalation may trouble the lungs or nervous system. Professionals working with hair products follow strict safety rules: gloves, ventilation, and immediate cleanup for spills. Occupational safety laws spell out safe-handling requirements, aiming to prevent long-term health trouble. That’s good, because repeated exposure over a career could create problems—a lesson passed down in lab safety briefings and beauty school classrooms alike.
Consumers have a role, too. Simple steps like reading labels, respecting application times, and rinsing thoroughly lower risk. For those with sensitive skin or a history of reactions, consulting a dermatologist or allergy specialist before using new hair dyes makes sense. Some groups push for less reliance on synthetic chemicals in cosmetics and call for biodegradable, plant-based alternatives. But for now, compounds like 4-Amino-m-cresol remain tools for precise, predictable results.
Research teams continue to search for safer ingredients, investigating how structural tweaks might keep color performance high but lower allergy risk. Increased transparency in cosmetics labeling helps people make better choices for themselves. What I’ve seen in my own life: information makes all the difference. Knowledge about what’s in a hair dye or lab reagent helps keep people safe, whether they’re sitting in a chair at the salon or experimenting in the lab after hours.
For people handling products at work or home, simple respect for the science behind each ingredient goes a long way. The path forward will likely involve both new chemical discoveries and better public understanding of the tools people already use every day.
4-Amino-M-Cresol, often found in hair dyes and some industrial processes, isn’t just another chemical in a bottle. I’ve spent time in labs enough to know chemicals like this command a certain respect. It’s easy to get comfortable around small bottles and faded labels; but if you slip into routine without thinking, you risk more than just a stain on your gloves. This compound can irritate the skin, harm the eyes, and release dust you don’t want in your lungs. Because it can get through the skin or get inhaled, proper handling makes a real difference in keeping yourself safe.
Pull on nitrile gloves before ever cracking a container open. Latex won’t always keep you safe with organic chemicals and cotton won’t cut it. Even a brief splash can burn or sensitize someone over time. Lab coats made from tightly woven fabric close off most routes for drips and dust. Safety goggles or face shields matter a lot with this one. You don’t want to test whether a splash really hurts your eye as much as the safety data says it does.
Working under a fume hood sounds tedious, but after years seeing colleagues cough through headaches or rashes, fresh air starts looking like a perk. Experiments or transfers should happen with ventilation running strong. Dust and tiny droplets ride air currents much farther than you’d think. A sealed process brought me peace of mind as much as it protected my skin and lungs.
Mixing 4-Amino-M-Cresol with other chemicals, especially strong acids or oxidizers, can turn a controlled process into a dangerous mess. Double-checking labels and MSDS sheets keeps you from making assumptions. Anything leftover—spills, empty containers, wipes—goes into waste containers clearly marked for hazardous materials. Flushing this stuff down the drain poisons more than just your local environment; it risks bigger fines and headaches for everyone using your building later.
Storing this chemical in a cool, dry spot, away from direct sunlight and incompatible substances, keeps it stable. Tight lids lock away fumes, and leak-proof bottles with clear labels help everyone stay aware. I’ve seen too many close calls from faded labels and half-sealed jars dumped on an open shelf. Taking the time to store things right saves hours later.
Training’s not just a box to tick on a workplace checklist. Walking through emergency showers, eyewash stations, and the location of clean gloves or masks prepares you for bad days before they start. Knowing exactly who to call and where to move cuts panic in a crisis. I make sure new people in the lab can find these places blindfolded. Spills happen. Eyes get splashed. Response time sets the outcome.
Sharing notes with coworkers about improved work habits or a fresh hazard you spotted reinforces a culture of care. Open communication lets good safety habits stick. Documenting incidents, even near-misses, keeps everyone learning together and brings hidden risks into the light.
Personal vigilance makes the difference. People who treat 4-Amino-M-Cresol as just another task waiting to be rushed rarely keep their skin, eyes, and lungs unharmed for long. Real learning happens not from rules alone, but from watching, sharing, and sticking to procedures every time—no matter how small the job.
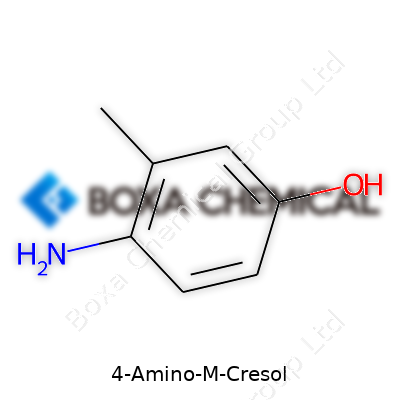
4-Amino-m-cresol sounds technical, but it's easy to understand. The chemical formula for this compound is C7H9NO. The molecule combines a benzene ring with a methyl group, an amino group, and a hydroxyl group all attached in a specific fashion. The “4-amino” part means an amino group (–NH2) sits on the fourth carbon of the benzene ring, while “m-cresol” shows a methyl group (–CH3) and a hydroxyl (–OH) on the ring, with “meta” indicating their positions in relation to each other.
Picture a hexagon, each point a carbon atom, all linked up. On one of those corners, put a methyl group. Move around the ring and set a hydroxyl group, then add the amino group opposite the methyl. That’s the layout. Chemists call it 4-amino-3-methylphenol. This matters because placement changes how this substance works in reactions and how it behaves in products like hair dyes and drugs.
I’ve noticed in my own chemistry days, especially working hands-on in the lab, that knowing exactly where to find each group on the ring saves a lot of headaches. For 4-amino-m-cresol, the structure shapes reactivity. With that amino group and hydroxyl group sitting across from each other, reactions for making dyes become simpler and more efficient. The methyl side—small but punchy—can make this compound more stable, less likely to break down if light or heat shows up.
It lines up as a go-to ingredient in oxidative hair dyes, delivering the punch of color that results in a lasting shade. While using it, people worry whether it’s safe. It’s good to know that if products using this compound meet legal standards, the risks to users can be managed. Scientists have tested it and regulators have set rules about how much can be used. Still, concerns exist. Allergic reactions or skin irritation sometimes show up. Clear labeling—and careful application—help reduce these hiccups.
On the environmental side, 4-amino-m-cresol shows up in the wastewater from factories and salons. If not managed, it can enter rivers and streams, where fish and plants could struggle. Over time, these molecules might build up and start changing the balance of the ecosystem. Many countries now ask producers to put safeguards in place, like improved filtration or finding ways to break down the compound before it leaves the factory. This helps protect water quality, making sure fields, streams, and neighborhoods stay healthy for everyone.
Education shifts the game. If more people—consumers and producers—stay alert to the chemical risks, misuse drops, health improves, and environmental impact drops. Manufacturers can invest in green chemistry to find or invent substitutes that offer the results needed, but break down faster and more cleanly outside the bottle. In labs, I’ve seen progress come when companies review their ingredient lists and swap out old compounds for less harmful options. As science and industry connect better, health and safety will improve for everyone who comes into contact with chemicals like 4-amino-m-cresol.
Anyone who spends a lot of time around chemicals will tell you more than once: the small details about proper storage matter. I learned this not from textbooks, but from a warehouse job in college, stacking barrels of compounds meant for dye and pharma labs. Bottles with hard-to-pronounce names, like 4-Amino-M-Cresol, came with paperwork listing hazard statements longer than a pizza menu. 4-Amino-M-Cresol is a powder used in making dyes and some pharmaceutical ingredients, but it doesn’t belong just anywhere on a shelf.
Moisture and heat are this compound’s worst enemies. If moisture sneaks in, clumping and breakdown can ruin a batch, sometimes even sparking a dangerous reaction. I still remember how our manager stressed the risk of storing sensitive powders next to windows or under leaky ceilings. We lost hundreds of dollars’ worth of chemicals from careless storage, and the worst part: exposure to degraded material has bigger consequences in the lab than just lost money.
So, a dry spot makes all the difference. In practice, that means fully sealed containers every time. Never leave the jar or drum cracked open, not even for a quick scoop. Stick with glass containers or chemical-proof plastic, always marked and always closed tight. Store the container in a place where the temperature stays steady—ambient room temperature works, but never near radiators or heat sources. I've seen coworkers try to save space by stuffing extras near steam pipes; what they get is a mess, not efficiency.
Direct sunlight speeds up chemical breakdown. It can fade labels, warp lids, and worst of all, it can mess with the purity of the powder. Any container should be opaque, or at least kept in a cabinet or storage box away from sunlight. And oxygen can react in ways that change the chemical itself. So airtight seals are just as important as keeping out moisture. If you’ve ever tried to reuse an old container with a sketchy seal, you know how fast air can creep in and compromise your material.
Labels aren’t just red tape. In the real world, people grab the wrong jar all the time—one slip, and someone gets hurt or ruins hours of work. Date everything, note the hazard level, and double up on the warnings. I print labels, then cover them with clear tape to stop them from smudging. It’s not pretty, but it sticks. My earliest mentor insisted on color-coded labels, and it prevented more than a few mix-ups.
Keep chemicals like this under lock or at least inside a cabinet with limited access. No one outside the team should stumble on them. I remember one audit, a spilled powder, and weeks of retraining because someone put a hazardous jar on an open shelf where the janitor could reach it. Access control isn’t paranoia; it’s learned from experience.
Spills happen, especially after a long day. Always sweep up with gloves and a mask—don’t give dust a chance to hit your skin or lungs. I have seen red hands more than once from someone in too much of a hurry. Dispose of waste in its own bag, label it, and get it out to hazardous waste pick-up soon. I never leave scrap chemicals in common bins; it’s an accident in the making.
Secure storage for 4-Amino-M-Cresol isn’t about following rules for their own sake. It’s about protecting people, product, and dollars. Real safety, at the end of the day, comes from caring enough to do every step right—even if you think no one is watching.
For years, hair dye users and workers in chemical industries have handled 4-Amino-M-Cresol. It’s a compound that shows up in hair color formulas as a dye intermediate. I’ve seen the appeal—deeper hair color, longer-lasting effects. But the safety of these chemicals matters to more than just the person applying the dye. The story stretches from factory floors to local rivers.
The biggest concern has always been what this compound does once it touches skin. Industrial safety data labels it as toxic in contact with skin or if inhaled, and that’s not just covering legal bases. Even tiny amounts absorbed through the skin can cause irritation. Reports from chemical handlers talk about rashes, redness, and sometimes more serious reactions. I’ve found studies hinting at longer-term risks, too, like damage to organs with repeated exposure. Despite those warnings, this compound isn’t as high-profile as lead or formaldehyde. People who color hair at home often don’t think twice about the long lines of ingredients on the bottle. But the hands-on folks—salon workers, factory operators—have seen the consequences up close.
Chemicals like this don’t just vanish after rinsing from hair. Wastewater treatment plants do a decent job breaking down dye chemicals, but they don’t catch everything. Runoff or leaks from factories end up in rivers, threatening aquatic life. Research has measured the effects on fish and insects—growth slows, reproductive cycles get disrupted, and sometimes tiny organisms just can’t survive. I once met a water quality specialist who saw local streams darken and wildlife populations drop after a factory started dumping untreated wastewater. It wasn’t always headline news, but the environmental cost sits in the background, racking up long-term damage that’s hard to reverse.
Regulators in the EU have started classifying 4-Amino-M-Cresol as a “substance of very high concern.” They require strict labeling and limit concentrations. The US lags behind, putting more burden on manufacturers to self-police. Over the years, I’ve noticed how some brands tout “ammonia-free” or “natural” on their labels. Often, they still use aromatic amines like 4-Amino-M-Cresol—there’s less transparency around these. Anyone choosing hair dye rarely gets a hazard sheet in the box. I’m a big supporter of letting people decide with full information. Stronger product labeling and easier-to-understand hazard ratings could help.
Dye-makers have started exploring plant-based options and less persistent chemicals. The shift is slow; color performance is tough to match without the old synthetic ingredients. Green chemistry innovators are looking for replacements with softer impacts. Last year, I talked with a small cosmetics firm investing in eco-friendly dyes—they struggled with costs, but their customers valued the switch. When buyers put pressure on brands, safer products start to make sense financially. For now, people working directly with 4-Amino-M-Cresol should use gloves, good ventilation, and washing stations. Communities living near manufacturing plants deserve the right to know what’s in their water and demand better controls. Public voices matter—they need to push for cleaner and safer alternatives, both for health and for the planet.
| Names | |
| Preferred IUPAC name | 4-Amino-3-methylphenol |
| Other names |
2-Hydroxy-4-methylaniline 2-Amino-5-methylphenol 4-amino-3-methylphenol |
| Pronunciation | /fɔːr-əˈmiːnoʊ ɛm ˈkriːsɒl/ |
| Identifiers | |
| CAS Number | 2835-97-4 |
| Beilstein Reference | **1209280** |
| ChEBI | CHEBI:16145 |
| ChEMBL | CHEMBL504188 |
| ChemSpider | 19540 |
| DrugBank | DB08797 |
| ECHA InfoCard | echa.europa.eu/infoCard/100.011.506 |
| EC Number | 202-424-3 |
| Gmelin Reference | 83235 |
| KEGG | C21077 |
| MeSH | D03HSF6GH7 |
| PubChem CID | 93007 |
| RTECS number | GF9600000 |
| UNII | E9O2L1W9EA |
| UN number | UN3456 |
| Properties | |
| Chemical formula | C7H9NO |
| Molar mass | 137.18 g/mol |
| Appearance | Light brown to brown solid |
| Odor | Odorless |
| Density | 1.108 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.64 |
| Vapor pressure | 1.15E-3 hPa (25 °C) |
| Acidity (pKa) | 10.23 |
| Basicity (pKb) | 7.85 |
| Magnetic susceptibility (χ) | -63.0e-6 cm^3/mol |
| Refractive index (nD) | 1.661 |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 109.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -40.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3221 kJ/mol |
| Pharmacology | |
| ATC code | D08AX01 |
| Hazards | |
| Main hazards | Harmful if swallowed or in contact with skin. Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. H317: May cause an allergic skin reaction. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements of 4-Amino-M-Cresol: "P261, P264, P280, P302+P352, P305+P351+P338, P310" |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | Flash point: 151.7°C |
| Autoignition temperature | Unknown. |
| Lethal dose or concentration | LD50 oral rat 1750 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1750 mg/kg (oral, rat) |
| NIOSH | RN 2835 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/m³ |
| Related compounds | |
| Related compounds |
4-Amino-2-methylphenol 3-Hydroxy-4-methylaniline 4-Amino-3-methylphenol |