The story of 4,6-Di-Tert-Butyl-M-Cresol, better known to some as BHT or butylated hydroxytoluene, traces back to discoveries in the early twentieth century connected to the fight against spoilage in fats and oils. In my chemistry lab days, talk about food shelf life always brought up BHT, a lipid antioxidant that broke ground for large-scale food preservation right as processed foods found their way onto household tables. Chemists saw that aromatic compounds with steric hindrance—meaning big, bulky groups attached to them—stopped unwanted oxidation impressively well. BHT’s value in lengthening the lives of everything from snack chips to industrial oils cemented its role in chemical manufacturing and consumer goods decades ago, and it remains a benchmark in the antioxidant space.
BHT earned a name for itself beyond food, packed into lubricants, rubbers, cosmetics, and even pharmaceuticals. It stops oxidation, which means it’s a shield against discoloration, rancidity, and breakdown under heat or light. Every corner of industry asks for antioxidants; folks who process plastics fight degradation, farmers want to keep their grains stored longer, and companies mixing up skin creams need to hold off the yellowing that ruins product appeal. My own experience cataloging chemicals in a warehouse taught me to expect BHT’s presence almost everywhere stabilization mattered, tucked into product labels and MSDS sheets where few end-users ever thought to look.
Physically, BHT appears as a white crystalline solid. It feels waxy to the touch, almost greasy, thanks to those bulky tert-butyl groups that insulate the phenol core from environmental attack. Its melting point hovers around 70°C, and it holds up in storage without decomposing, making it a practical choice for shippers and manufacturers who need long shelf life. BHT dissolves easily in organic solvents like ethanol or toluene. Odor is faint and slightly aromatic, nothing overpowering, so it doesn’t taint the flavor or scent of packaged goods at used concentrations. Chemically, the structure centers on a methylphenol ring: the backbone for radical scavenging, allowing BHT to neutralize peroxides and other reactive species before they spark chain reactions in susceptible materials.
Quality standards for BHT fall under tight controls due to its widespread use in foods, pharmaceuticals, and industrial applications. Purity usually exceeds 99 percent, with careful monitoring for byproducts or contaminants like heavy metals. Labeling requirements come down from regulatory authorities worldwide, such as the US FDA or the European Food Safety Authority, spelling out not only allowed concentrations—typically capped around 0.01 to 0.02 percent by weight in consumables—but also mandatory ingredient disclosures on packaging. Across my work, I’ve seen safety data sheets include signal words for chemical handling, identification numbers like CAS 128-37-0, and recommended storage practices to keep the product dry, away from direct sunlight or strong acids and oxidizers.
Industry manufactures 4,6-Di-Tert-Butyl-M-Cresol by alkylating m-cresol with isobutylene, a process that takes place under acidic catalytic conditions. Large reactors combine the reactants at controlled temperatures and pressures, monitoring progress until conversion reaches a target yield. The art of scaling up from small bench synthesis to large-scale production lies in balancing reaction speed, catalyst regeneration, and purification steps. Crystallization and filtration pull the final BHT crystal from reaction mixtures. Many times, a project will focus on making the process safer or greener, reducing waste acids or volatile organics. Plant engineers watch for equipment corrosion and work to minimize energy input per kilogram produced; every bit of efficiency counts now that sustainability and economics share center stage.
Chemists regard BHT as a platform molecule for innovative modification. Its phenolic oxygen doesn’t just donate electrons to catch free radicals; it also acts as a launch pad for further chemical derivatization when transforming BHT into specialty additives needed for paints, resins, or custom polymers. In some research labs, I joined teams testing oxidative coupling or sulfonation to introduce new properties or improve solubility for niche applications. Under carefully chosen conditions, halogenation or acylation reactions build upon the BHT scaffold. Those tert-butyl groups, once in place, provide a protective shield that limits unwanted side reactions, helping ensure selectivity and making BHT a reliable chemical base for R&D across material science fields.
Anyone working with chemical inventories soon realizes the gamut of names attached to 4,6-Di-Tert-Butyl-M-Cresol. Industry folk call it BHT most often, but synonyms include Butylated Hydroxytoluene, 2,6-Di-Tert-Butyl-4-Methylphenol, and numbers like CAS No. 128-37-0. Product grade names or commercial labels run from Ionol to Embanox and Additin, depending on country or manufacturer. The many names lead to confusion; knowing how to cross-check specifications, purity, and regulatory status helps avoid slip-ups in ordering or compliance, as anyone sifting through international shipments learns quickly.
Handling BHT calls for a respect for its low but real toxicity profile, common to many industrial organic compounds. Long-term daily exposure, whether by inhalation of dust in production plants or skin contact during batching, triggers workplace controls you’d expect: respirators, gloves, eye protection, and regular air monitoring. Storage procedures minimize accidental fire, since BHT will burn if ignited, though its risk sits lower compared to solvents or metallic reducing agents nearby. Disposal challenges involve collecting and treating waste material to prevent environmental leakage, as surface water ecosystems respond poorly to high phenolic loads. Training and best-practice guidance drive safe use, while regulatory bodies enforce limits on allowable airborne concentrations and post-exposure cleanup.
BHT shows up in products that protect, preserve, and stabilize. In my early career unpacking analytical standards, I spotted BHT in everything from cereal samples meant for lipid analysis to test kits for monitoring shelf life. Companies use it to fortify animal feeds against nutrient loss and add it to paints and lubricants to delay aging. In many regions, people ingest trace amounts daily in foods with added fat content, since manufacturers rely on BHT to keep oils fresh. Cosmetic formulations depend on it for preventing oxidative color changes. Synthetic rubber compounds use it to maintain elasticity and extend product utility. BHT’s utility keeps growing, as industries develop smarter packaging and storage to fight off the relentless push of oxygen and time.
BHT attracts continuing R&D, both for its antioxidant power and for potential upgrades. Scientists leverage advanced computational chemistry to tune the phenolic backbone, looking for derivatives with better biodegradability or lower bioaccumulation. Analytical chemists refine testing protocols to measure minute levels of BHT residues in foods and tissues, a nod to both regulatory evolution and consumer demands for traceability. Some biotechnologists study natural alternatives that mimic BHT’s properties, searching for greener drop-in replacements. Meanwhile, researchers map out BHT’s behavior inside packaging systems, paints, and even pharmaceuticals to stretch shelf life further while keeping safety profiles strong. Grants and public–private partnerships often steer the search toward more sustainable chemistry and circular economy models where every molecule counts.
The toxicology of BHT drives passionate debate in many circles, and I learned early to respect both the science and the social context surrounding it. Lab studies in rodents have documented dose-dependent liver and kidney changes, with high-level exposures leading to cellular and metabolic disruption. Most authorities, including the FDA and EFSA, set acceptable daily intake (ADI) limits with wide safety margins based on animal studies and careful extrapolation to humans. Rigorous peer-reviewed meta-analyses usually report that low intake poses minimal risk, though questions still linger on potential cumulative effects or endocrine influence. Follow-up studies chase reported allergic reactions, genotoxicity, or possible links to hyperactivity, but concrete evidence remains rare at regulated use levels. The broader push in toxicology research now includes advancements in in vitro screening and computer modeling to predict metabolic outcomes and long-term impact, helping push the industry to safer, more thoroughly vetted use scenarios.
Shifting consumer trends, regulatory pressures, and green chemistry advances all point to an evolving future for BHT. Industries recognize that legacy antioxidants like BHT, with decades of proven value, must adapt to stricter purity requirements, environmental protections, and consumer transparency. I’ve watched startups and established producers alike expand into alternate antioxidants, making way for blends that promise lower environmental persistence without sacrificing effectiveness. Nonetheless, BHT’s role in safeguarding product integrity stands strong, and replacement efforts still gravitate toward its unique combination of cost, availability, and proven durability. The coming years will see more precise analytical detection, greater focus on lifecycle assessment, and, possibly, tactical phasing out in non-essential uses as safer, renewable alternatives emerge. The chemical, regulatory, and public health communities continue to weigh long-term health data with practical needs, searching for solutions that respect ecosystems, people, and ongoing innovation.
Most grocery store shelves carry products that depend on a little chemistry for a longer life. 4,6-Di-Tert-Butyl-M-Cresol, often known in labs and factories as BHT, is an essential player here. Pick up a bag of chips, open up a bottle of vegetable oil, and there’s a decent chance this antioxidant is quietly protecting the food from turning rancid. Without this compound, shelf life shrinks and food quality drops long before you get it home.
Growing up with a parent who worked in food manufacturing opened my eyes to the problem of spoilage. Fats and oils break down fast when air and light get to them. They smell awful, taste even worse, and their nutritional value slides. BHT slows that decay. Science backs this up: research shows its molecular structure interrupts free radical reactions responsible for spoiling fats, keeping food fresh for weeks, sometimes months, longer.
Beyond kitchens and pantries, 4,6-Di-Tert-Butyl-M-Cresol stands guard in products most people use every day without thinking twice. Personal care items—creams, deodorants, lipsticks—contain fats and oils too. I once dug through the ingredients lists on my own bathroom shelf and spotted it listed in everything from shaving cream to sunscreen. Skin products stay more stable and don’t go off as quickly thanks to this antioxidant.
Manufacturers put a lot of work into each formula. The last thing they want? A shipment recalled because the cream separated, smelt odd, or lost effectiveness halfway through its shelf life. Here, BHT shows up not for flavor, but for stability. Cosmetic scientists, as noted in journals on formulation safety, continue to rely on BHT because alternatives often fail under heat or long storage.
Industry gets even more creative with this chemical. Rubber and plastic products use 4,6-Di-Tert-Butyl-M-Cresol to keep polymers from falling apart when exposed to sunlight and oxygen. Car tires, electrical cables, appliance housings—anything that combines plastics with long-term durability relies on slowing down the chemical changes caused by the environment.
Think of the wiring in your car or devices at home. When plastics degrade, wires get brittle and break—a safety problem. Refineries and plants that produce rubber parts rely on antioxidants like this to extend their products’ working life. Huge amounts of money hang in the balance: fewer replacements mean lower maintenance costs and safer products.
Some folks worry about the long-term impact of exposure, especially through food and personal care products. While agencies like the Food and Drug Administration review safety data and set limits for use, some studies spark debate about higher levels or routine daily intake. So far, evidence points to safety at current limits, though ongoing research always has room for surprises.
For those wanting to avoid it, reading labels or choosing products labeled “antioxidant free” offers some control. Companies keep looking for substitutes, especially natural antioxidants like tocopherols (vitamin E), but the results don’t always match up in every application. Maintaining performance while addressing public health questions keeps chemists busy and drives innovation in cleaner and greener antioxidants.
People working in research, chemical storage, or manufacturing run into substances like 4,6-Di-Tert-Butyl-M-Cresol, sometimes called BHT. This antioxidant sits in plastics, rubber, and food packaging. While it keeps products from degrading, the risks get ignored until skin stings or a chemical scent lingers too long. Stories from experienced technicians show why every step matters, no matter how boring the safety routine seems.
Labs stock gloves, goggles, masks, and lab coats for a reason. A friend once supposed he’d be fine measuring BHT bare-handed, since "it’s just an antioxidant." The redness and itching on his palms lingered for days. The chemical leaves behind an oily film that sinks into skin, and on a warm day, your hands soak it up faster than you expect.
Safety data from trusted sources like the CDC lists skin, eye, and respiratory irritation as frequent complaints. Splash goggles cut down on costly trips to the eye-wash station. Nitrile gloves keep absorption to a minimum. Good airflow keeps fumes from piling up in your lungs—especially if you’re moving powders or heating a sample. Chemical labs live by the fume hood’s hum, not for comfort, but because one forgotten fan can push everyone’s luck.
Nobody likes keeping a tidy bench, but pay attention to where the compound spills and who cleans up next. BHT often looks like a simple off-white crystal, so small spills can blend into surfaces. If it stays out, traces can transfer onto lunches, door handles, and paperwork. Paper towels in a sealed bag land in special disposal bins. Soap and water clear BHT off glassware—never skip the wash, even if you’re short on time.
Good labeling keeps surprises out of the picture. Mark every bottle with contents and hazard reminders. I’ve watched interns reach for mystery jars, trusting the previous shift. A quick label and date save much bigger headaches than a five-minute chore.
Professionals treat safety talks as practical check-ups, not chores. Younger workers learn by watching cautious mentors double-check their personal protective equipment, never letting familiarity get in the way of safe handling. Open doors for questions and don’t mock rookie mistakes—real learning comes from working things through, not pretending nothing happens.
If anyone feels dizzy or their nose tingles during work, they know to step outside quickly. Plenty of accidents happen from trying to finish work instead of asking for help, or hiding spills for fear of embarrassment. The best teams talk early and often about every mistake, so nobody repeats it.
The industry keeps pushing for safer alternatives and better containment tools. Automated scoopers, reach tools, and improved packaging help cut down direct handling. Regular audits catch weak spots in training or worn-out gear. No routine gets too old to skip, because the next shortcut can cause real harm.
People using chemicals will always face risks, but every lesson from seasoned workers proves that details matter. No bravado, no guesswork—just trust in the simple steps that keep the work safe for everyone.
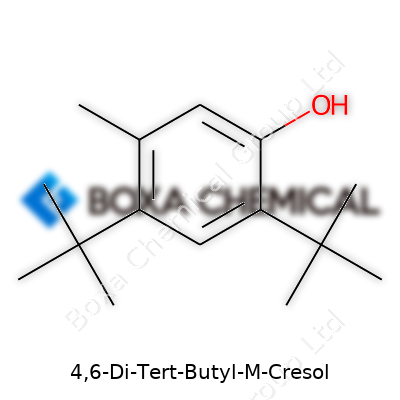
When I look at 4,6-Di-Tert-Butyl-M-Cresol, I see more than a tongue-twister. The name tells a story about what sits on its aromatic ring. This compound stands out because of those big tert-butyl groups attached at the 4 and 6 positions. Under the microscope, its chemical formula shows up as C15H24O. That’s a total of fifteen carbons, twenty-four hydrogens, and a single oxygen holding down the fort.
Chemists who care about structure might draw it like this: start with a benzene ring, a simple six-carbon loop. At the 1-position, you find a methyl group (a -CH3 tail), and hooked onto carbon 3 sits a hydroxyl group (an -OH). Tert-butyl groups, more like molecular boulders, claim their spots at positions 4 and 6 along the ring. The structure doesn’t dance around. Its full IUPAC name, 2-methyl-4,6-di-tert-butylphenol, fills in those details. In shorthand, folks often call it BHT or butylated hydroxytoluene, a mouthful turned common in the food and cosmetics world.
I’ve seen plenty of preservatives over the years, but BHT keeps landing on ingredient labels for a reason. Those hefty tert-butyl branches stick out, protecting the core of the molecule. That bulk helps BHT block reactions with oxygen, which slows down the process that makes oils and fats go rancid. I’ve watched crackers, cereals, and even makeup last longer because of it.
The location of the hydroxyl group brings another layer to its power. The -OH doesn’t just hang around. It donates a hydrogen atom—essentially a shield—when free radicals pop up. These wild molecules can damage cell structures or spoil food. Handing over a hydrogen to a radical kicks off a chain reaction that stops the damage in its tracks. That’s not a small win, especially where shelf life and safety go hand in hand.
It’s tempting to pop BHT into everything, but I learned from scientists in toxicology labs that balance matters. Small amounts usually stay safe, but big doses raise eyebrows. Animal studies point to possible liver effects if folks eat way more than the recommended daily levels. The FDA and European Food Safety Authority have dug deep into the details, placing strict limits on how much makes it into food products. From what I’ve seen in regulatory paperwork, the exposure most people get falls well below any risky zone.
Modern life requires some chemistry. I’ve talked to food manufacturers hunting for options to meet growing consumer preferences for “clean labels.” Some switch to mixed tocopherols—forms of vitamin E—or rosemary extracts, but those replacements come with tradeoffs. They don’t always play nice with every type of fat or every shelf-life target. Transparency helps customers make choices about what they eat. Regulations demand clear labeling, so it’s easy to spot BHT on the back of a box.
To sum up, 4,6-di-tert-butyl-m-cresol packs a chemical punch in a small package: a compact phenol with heavy protecting groups, built to ward off spoilage. The science sits right on the label, not just in the lab. Chemistry built wisely can shore up food security and safety, as long as transparency and research stay front and center. Facts, not trends, lay a solid foundation for trustworthy choices at the grocery store and in product development.
A lot of folks in chemistry labs and industry have stories about overlooked storage. Nobody forgets the time a drum sweated out in a humid warehouse or when something leaked thanks to a cracked lid. 4,6-Di-Tert-Butyl-M-Cresol (BHT) might sound like a mouthful, but most people know it as an antioxidant additive found in rubber, plastics, and sometimes food. Storing it with a loose approach can trigger real problems. Let’s get more specific.
People reach for whatever drum, bag, or bottle is close at hand, but BHT calls for a tough, well-sealed container. Plastic buckets eventually crack; thin plastic lets vapors seep. Heavy-gauge steel or high-density polyethylene work best. It comes as a pale, crystalline solid, which means moisture is the enemy. I’ve seen carelessness lead to caking that makes a product useless—nobody wants to chip chunks out by hand, then guess on the dose.
Seal the container tight after each use. Replace gaskets or lids at the first sign of wear. That close attention keeps the powder dry and free-flowing.
Lots of industrial warehouses run hot in summer, but heat breaks down BHT. Store it under 25 degrees Celsius—roughly room temperature. Sudden spikes speed up oxidation, so when air conditioning fails or the sun hits a window, quality dies off and impurities start to form.
Direct sunlight does more than warm things up. UV light nudges chemicals toward instability; you don’t want breakdown products in your antioxidant. Most packaging comes painted or tinted for a reason. You can stack boxes against a north wall or use a UV-blocking tarp, but routine sneaks in. Place thermometers around the shelves and check weekly.
BHT looks harmless, but reactive stuff in the next aisle can stir up trouble. Store it away from acids, peroxides, or anything that might trigger a reaction. A good rule—keep an aisle’s width or solid barrier between organics and oxidizers. Spills from leaky shelves become less terrifying when chemicals aren’t jumbled together.
A real-life example sticks with me: a splash of cleaning acid nearby once led to a cloud of strange smells. It took days of testing to figure out if the batch was still safe. Keeping supplies clustered by type sounds fussy, but it dodges emergency phone calls and product waste.
Crib sheets with bold label dates, hazard symbols, and your warehouse’s contact details turn ordinary bins into safe supplies. People sometimes ignore these steps until something goes wrong. Nobody likes tracking expiry dates—digital reminders and regular checks cut that hassle down.
Sweeping up powder spills with regular brooms leaves fine dust everywhere. Vacuum with HEPA filters and designated brushes work cleaner and protect lungs.
Storing BHT right feels thankless until something goes awry. Start with the basics: tough containers, consistent cool storage, keep products separated, and remind everyone about routine checks. Those habits save time, money, and keep unwanted reactions at bay—which matters just as much in a school storeroom as it does in a global supply chain.
4,6-Di-tert-butyl-m-cresol isn’t exactly a household name, but the things it prevents—like old cooking oil turning rancid or plastics breaking down under sunlight—show up almost everywhere. It goes into food packaging, lubricants, rubber, even cosmetics. Manufacturers use it to slow oxidation, so products look and perform better for longer. That sounds convenient for business and for people wanting stuff that lasts, but there’s more beneath the surface.
Chemists have learned plenty about 4,6-Di-tert-butyl-m-cresol, usually tagged as BHT’s chemical cousin. Studies in animals give a mixed bag—sometimes these compounds have caused liver and kidney issues after long exposure to high doses. The International Agency for Research on Cancer (IARC) hasn’t called it a definite carcinogen, but scientists highlight its tendency to disrupt hormone systems and tinker with enzymes. It's not in the same league as the most notorious chemicals, but over time, small doses may build up.
The bigger challenge hits lab workers or factory crews handling the raw powder or liquid. Without good ventilation or protective gear, skin or lung exposure brings up short-term irritation. Regulatory agencies—like the European Chemicals Agency—have flagged it for long-term aquatic toxicity, urging limits on how much can show up in waterways. The U.S. EPA lists limits for workplace airborne exposure, but no one expects consumers to face those levels just from finished products.
In rivers or lakes, this chemical doesn’t break down fast. Its molecular structure, designed for stability, resists sunlight and bacteria. Water treatment plants struggle to filter it. Chemicals like these pile up in sediments, sometimes winding up in small fish. They can move up the food chain and wind up in birds or predators later on. Europe classifies it as “very toxic to aquatic life with long lasting effects.” Researchers have tracked persistence and found traces in sewage sludge spread on fields. Over time, a buildup becomes hard to ignore.
A simple step for anyone is reading labels—on food packaging, cleaning agents, or cosmetic items—though the name may hide under E321 or “antioxidant.” Choosing products labeled as “BHT-free” or using alternatives like tocopherols (forms of vitamin E) helps. Some companies have already switched because of public pressure and changing guidelines.
The problem isn’t solved by individual choices alone. Stricter rules on commercial usage, stronger wastewater treatments, and more transparency from industry leaders make the bigger impact. Testing alternatives that break down safely matters; not just swapping one synthetic for another with the same risks. Several firms in Europe have turned to natural preservatives, showing the shift is possible.
Science keeps moving, and public concern shouldn’t wait for an outcry. Regular review of chemicals in everyday items, funding unbiased research, and honest communication—those steps keep both people and the environment safer. There’s no quick fix, but a mix of smart regulation, company responsibility, and good old curiosity about what sits in the products people use gives more control over these risks.
| Names | |
| Preferred IUPAC name | 2,6-di-tert-butyl-4-methylphenol |
| Other names |
2-Methyl-4,6-di-tert-butylphenol BHT Butylated Hydroxytoluene DBPC Ionol |
| Pronunciation | /ˈfɔːr sɪks daɪ tɜːrt ˈbjuːtɪl ɛm ˈkrɛsɒl/ |
| Identifiers | |
| CAS Number | 128-37-0 |
| Beilstein Reference | 2051440 |
| ChEBI | CHEBI:31355 |
| ChEMBL | CHEMBL1424 |
| ChemSpider | 13236 |
| DrugBank | DB03255 |
| ECHA InfoCard | 100.014.265 |
| EC Number | 128-37-0 |
| Gmelin Reference | 61357 |
| KEGG | C06535 |
| MeSH | D001937 |
| PubChem CID | 31404 |
| RTECS number | EO3325000 |
| UNII | W83C517V3Q |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C15H24O |
| Molar mass | 220.35 g/mol |
| Appearance | White crystalline powder |
| Odor | odorless |
| Density | 1.05 g/cm3 |
| Solubility in water | Insoluble |
| log P | 5.0 |
| Vapor pressure | 0.000133 hPa (25 °C) |
| Acidity (pKa) | 11.6 |
| Basicity (pKb) | 10.51 |
| Magnetic susceptibility (χ) | -77.0e-6 cm³/mol |
| Refractive index (nD) | 1.507 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 172.0 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -392.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7110 kJ/mol |
| Pharmacology | |
| ATC code | A05BA02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. May cause damage to organs through prolonged or repeated exposure. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | 127°C |
| Autoignition temperature | 410°C |
| Lethal dose or concentration | LD50 Oral Rat 890 mg/kg |
| LD50 (median dose) | LD50 (median dose): 890 mg/kg (oral, rat) |
| NIOSH | DU8050000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of 4,6-Di-Tert-Butyl-M-Cresol: "TWA 10 mg/m3 (total dust) |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | Unknown |