The story of 4,4'-Thiobis(6-Tert-Butyl-M-Cresol) traces a path through the evolution of polymer science. Chemical companies in the mid-20th century set out to answer industry’s call for new antioxidants that could preserve materials exposed to tough conditions. Early research into phenolic compounds highlighted how sulfur bridges could make antioxidants more stubborn against breakdown. Chemists built up the molecular backbone with tert-butyl groups, which offered not just bulk but also stability. During a period when synthetic rubbers and plastics started to overtake natural materials, this compound stood out because it tackled two major threats—heat damage and oxidation. Over the decades, incremental improvements kept it in the toolkit of engineers and materials scientists, especially as performance standards for tires, cables, and gaskets inched upward. I grew up seeing tires replaced less often, in part because advances like these stretched out product lifespans, which always fascinated my mechanical engineer father.
4,4'-Thiobis(6-Tert-Butyl-M-Cresol), often slotted into antioxidant categories as “Antioxidant 300” or similar names, earned a reputation for its efficiency in stabilizing synthetic polymers. Industrial suppliers ship it out as a pale yellow powder, and those who handle it learn quickly that dust control and good storage protect its quality. Use often gravitates to rubber compounds with high vulnerability to heat, air, and sunlight, like those found in high-speed tires or heavy-duty conveyor belts. Performance here matters both to safety and economics. The material reduces cracking and preserves elasticity under repeated stress. It functions as a chain-stopper, guarding polymer chains from scission—the term for cleaving them apart—and that role resonates across industries, from automotive components right through to wire insulation. Each time I visited a wire factory, I could feel the mix of chemical expertise and hands-on practicality that kept these additives among the most trusted in the business.
Physically, the compound presents as a crystalline powder, usually pale yellow, stable at room temperature and resistant to moderate humidity. Its molecular structure—two cresolic rings joined by a sulfur atom, each ring flanked by robust tert-butyl groups—creates significant steric blocking, which helps shield the active sites from oxidative attack. The melting point usually falls in the range of 140–145°C, high enough to survive most in-process temperatures in rubber compounding. Solubility runs low in water, much higher in organic solvents like benzene or acetone. That can raise challenges for those mixing or formulating in large reactors, since you have to play matchmaker between solubility and dispersion. In reactivity, the phenolic hydrogens act as sacrificial agents, offering up their electrons to trap peroxy radicals, which are the main culprits in polymer aging. Getting hands-on with these materials over the years, I learned that these tricky physical characteristics influence not just lab work but the design of industrial-scale processes too.
Suppliers publish detailed certificates of analysis with each batch. Purity levels typically run above 98%, with moisture content held below 0.2%. Ash content, measured after burning off organics, remains very low—usually less than 0.1%—ensuring that residues do not affect finished products. Particle size specifications matter in mixing and dispersion; the more homogeneous the powder, the more consistent the performance. Manufacturers label product drums with UN-compliant hazard codes, batch numbers, and expiry dates, reflecting both the material’s chemical sensitivity and the strict regulatory oversight found in chemical handling. My work in quality assurance hammered home just how much these data points matter: catching an off-spec batch early can mean the difference between a successful production run and a costly recall.
The classic synthesis draws on the reaction between 2,6-di-tert-butyl-4-methylphenol and sulfur dichloride. This method, which dates back to postwar industrial chemistry, leverages the nucleophilic substitution capability of the phenol under basic conditions. The process typically runs in chlorinated solvents under strict temperature control to avoid unwanted side-products, especially di-ortho substitution or incomplete sulfur bridging. Work-up to separate and purify the final product often relies on re-crystallization, which can define final purity and appearance. Years spent troubleshooting organic syntheses made me appreciate how finicky these steps can be—one misstep in timing or temperature delivers impurities, which sabotage product performance before it even leaves the lab.
Standard reactivity revolves around the phenolic hydroxyls, which serve as radical scavengers, but synthetic chemists keep an eye out for side reactions that could affect color or stability. Sulfur atom in the central bridge can attract attention if severe processing or aggressive oxidants come into play, sometimes forming sulfoxides or sulfones under harsh conditions. Chemical modifications occasionally tweak ring substituents to tune solubility, but for most applications, the tert-butyl groups remain untouched because their sheer size protects the molecule’s core. Material scientists keep exploring whether small tweaks might improve compatibility with new polymer blends or foster better processing properties; sometimes, even a subtle change offers a jump in performance.
Across catalogs and safety data sheets, you’ll run into several synonyms—Antioxidant 300, BIS(6-tert-butyl-m-cresol) sulfide, and 4,4'-Thiobis(6-tert-butyl-meta-cresol) topping the list. CAS numbers provide the most reliable universal reference, cutting through the confusion of trade names and regional labeling conventions. Major producers in Japan, Germany, and the United States each attach their own codes and commercial brands, which leads to a mix of short-hand product titles. Anyone working in procurement gets used to double-checking these identifiers to avoid mix-ups with similar antioxidants; I found plenty of stories in supplier meetings where mislabeling led to warehouse headaches and delivery delays.
Handling this antioxidant demands respect for both occupational health and environmental regulations. Dust can irritate eyes and respiratory systems, so factory workers use local exhaust ventilation, gloves, and protective eyewear. Regulations stemming from OSHA and REACH dictate exposure limits and mandate reporting for bulk transit and spillage. Standard storage—cool, dry, away from sunlight—slows degradation and helps avoid sticky clumping, a problem that gums up feed hoppers. Emergency response plans form part of every handling site’s safety protocols, shaped by both internal procedures and national regulatory pressure. From personal experience in chemical plants, these aren’t just paperwork—they underpin both worker safety and environmental stewardship, with surprise inspections more common than some would think.
Biggest demand comes from the rubber industry, with a particular focus on car and truck tires facing high thermal and mechanical stress. Cable jackets, gaskets, vibration dampers, and a wide range of elastomer products rely on this antioxidant to block UV-driven or thermal oxidation. Some rigid plastics, especially those used in long-life electrical insulation, pick up added protection through small inclusions of this material. Since every application brings its own idiosyncratic blend of stresses—exposure to hydraulic fluids, ozone, heat—engineers and compounders experiment to see which additives work best in real world use. Years of hearing from technical service reps about field returns impacting major brands drove home how essential these tailored formulations prove in practice.
Ongoing research chases both performance and sustainability. On one front, chemical engineers investigate derivative structures aiming for enhanced radical trapping or faster action at lower use levels. Academics study compatibility with “green” rubber blends or new polymers emerging in wire and cable insulation. Environmental scientists interrogate breakdown products for clues about long-term safety in landfill or incineration. Some efforts push for bio-based feedstocks or improved process engineering to minimize byproducts and energy costs. Collaborations between industry and university labs shape the pipeline for next-generation antioxidants; I’ve seen projects combining sophisticated modeling with good old-fashioned bench chemistry to probe margin gains in both safety and effectiveness.
Toxicologists pore over both acute and chronic effects. Standard studies on rodents assess ingestion, inhalation, skin exposure, and environmental fate. So far, typical hazard rankings fall below those of heavy metal-based stabilizers, though the compound earns a “handle with care” tag for dust inhalation and potential liver impacts at heavy, chronic doses. Regulatory agencies keep a close watch and update guidelines from time to time as new data arrive. Waste handling, especially incineration or landfill leachate risk, attracts ongoing scrutiny. Industry-funded and independent tests collaborate to pinpoint safe use levels, reflecting both public health expectations and the unavoidable uncertainties that come with modern chemical production. Over the years, the focus on transparency has grown, and material safety data sheets get updated with every significant new research finding.
The future for 4,4'-Thiobis(6-Tert-Butyl-M-Cresol) will be shaped by three main threads: regulatory shifts, environmental pressures, and demand for ever-more durable polymers. As governments tighten restrictions on chemical additives and public awareness over synthetic antioxidants grows, pressure increases to document both short- and long-term safety. Development of recycled and bio-based elastomers offers new testing grounds for antioxidant designs, and companies big and small look for molecules that work reliably in these novel systems. Automation and digitalization in manufacture could add another layer of control and traceability, helping assure regulators and downstream users alike. Experience tells me that the humble antioxidant remains a linchpin—manufacturers and consumers rely on hidden chemistry to keep materials working longer, safer, and more sustainably. As long as technology evolves, the need for rigorous, honest research on compounds like this one won’t fade away.
Antioxidants affect our daily lives a lot more than most people realize. I remember back in my manufacturing days, I was shocked at how quickly a batch of polymer could yellow and break down if nobody remembered to add the right stabilizer. The culprit is often invisible—oxygen from the air attacking the polymer chains. 4,4'-Thiobis(6-Tert-Butyl-M-Cresol) (often called TBBC or antioxidant 300) stands out as one of the compounds that defend materials from this kind of slow destruction.
In factories where plastics and synthetic rubbers get shaped into everything from hoses to garden chairs, TBBC shows its value every day. Think of the last time you saw a brittle car dashboard or a faded plastic pool toy. Sunlight, heat, and air work together to break down polymers at a molecular level. It’s not just an aesthetic problem—broken, discolored parts signal real loss of safety and durability. TBBC slows that degradation by sacrificing itself in chemical reactions that would otherwise attack the polymer.
Rubber for tires, seals and gaskets face years of mechanical stress and weather. Manufacturers count on TBBC because it's strong against oxidation at high temperatures. I’ve seen labs push these materials hard to predict if a tire or hose might crack early. Results show that products with TBBC last longer in the field. Manufacturers trust its performance, particularly in engine mounts and automotive weatherstripping.
Plastic electronics housings, cables and tubes all take a beating over years. The electrical industry cannot afford short circuits or hazardous failures caused by brittle, crumbling insulation. TBBC helps maintain flexibility and stability. I’ve talked to cable engineers who say that without additives like TBBC, some critical wiring wouldn’t pass safety codes.
Beyond plastics, some industrial lubricants and fuels draw on TBBC as well. Gear oils spend a lot of time facing pressure and heat inside machinery. The antioxidant keeps oil from gumming up or breaking down. When turbines run for months at power plants, or when delivery trucks pile up thousands of miles, improper lubricant oxidation can cause catastrophic equipment damage and business losses. Companies use TBBC to extend the life of fluids and keep everything running smoothly.
No one wants additives leaching into food, water, or soil. TBBC, like many industrial chemicals, has raised concerns about bioaccumulation and possible toxicity. I remember a local recycling program struggling to find dependable ways to detect and manage chemicals like this during processing. Researchers and regulators are cracking down by toughening standards and launching more studies, especially for uses where human exposure might happen. Many producers now limit TBBC in applications involving direct human contact.
Chemical safety takes teamwork—scientists, plant operators, regulators, and everyday people all playing a part. Staying on top of research, developing safer alternatives, and strengthening recycling and waste management all help reduce risk. Some global companies already invest in advanced monitoring and safer substitutes, showing that environmental responsibility and product reliability can go hand-in-hand.
Every time you see a durable tire, a flexible cable, or a faded playground slide that’s still unbroken after years in the sun, chances are, antioxidants like TBBC played a role behind the scenes. Chemicals like this help modern products last longer and stay safer, but their use demands respect for health and environmental risks. Experience tells me that open information, better monitoring, and targeted innovation unlock better solutions for everyone who relies on these everyday materials.
Plenty of folks in manufacturing and lab environments bump into tongue-twister chemicals every day, and 4,4'-Thiobis(6-Tert-Butyl-M-Cresol) is no stranger in the antioxidant world. Chemists turn to it for its ability to protect plastics and lubricants from degrading. Before anyone grabs a bag or bottle marked with this name, it’s smart to know what science and safety data reveal about handling it.
From my own days training in industrial hygiene, the Material Safety Data Sheet was always the first thing we checked. This molecule, often shortened to TBM-6, carries a “harmful if inhaled or ingested” warning. There’s also skin and eye irritation risk. Not exactly something you’d let float around a poorly ventilated space. Repeated skin contact has even led to allergic reactions in some workers.
Here’s where the science grounds things. Animal studies show that repeated exposures over a long haul might add stress to the liver and kidneys. OSHA and the European Chemicals Agency rate it as requiring extra care. The Centers for Disease Control names gloves, goggles, lab coats, and ventilation as the must-have gear. We aren’t talking about uranium, but it’s not baking soda either.
In many plastics factories, handling rules for TBM-6 fall in line with ones for similar phenolic antioxidants. Most use enclosed systems or demand fume hoods. Good companies train up everyone who handles it and run mock spill drills for a reason. The point is to keep airborne dust down and skin covered. Companies that skip these things see higher worker complaints—burning eyes, skin rashes, scratchy throats—and sometimes big fines from regulators.
I watched a mid-sized lab swap out open bench-top containers for sealed weighing stations after three technicians developed rash outbreaks. None became seriously ill, but all got a lesson in how invisible powders can get real close, real fast.
The risk in handling TBM-6 really boils down to respect, training, and workplace controls. Anyone handling kilograms each day should have full PPE and work in ventilated zones. Storage matters, too. This chemical breaks down in high heat and sun, releasing even more irritating compounds, so it should stay cool, out of the sun, and away from acids.
Companies take this stuff seriously because liability grows when corners get cut. The Environmental Protection Agency tracks spills for exactly this reason. It’s not just about worker health—the wrong exposure can cause environmental messes, too.
A lot gets avoided by assigning a single safety coordinator who checks air quality, reviews in-house incident reports, and trains the team upfront. Spill kits and wash stations don’t collect dust in responsible businesses, and regular updates from suppliers keep crews aware of the latest handling guidance. This matters, because both worker health and product quality depend on sharp protocols.
Long story short, TBM-6 doesn’t belong in uncontrolled, unlabeled, or poorly ventilated spaces. Handle with proper gear, keep the workspace clean, and stay current with safety guidance—and the risk goes way, way down. I wouldn’t want to work in a place that treats it like sugar or flour, but I’d trust a shop that puts safety first every time.
Anybody working with chemical additives in plastics and rubber knows headaches come from missing the mark on storage basics. 4,4'-Thiobis(6-Tert-Butyl-M-Cresol), a phenolic antioxidant, isn’t just another line on a spreadsheet—it’s a substance that reacts to mishandling. Keeping it in top condition protects both chemical performance and the people on your team.
This compound shows real sensitivity to air, moisture, and heat. As the years pass, I’ve seen what happens when a drum of stabilizer sits a few days too long by an open loading dock. A little bit of humidity sneaks in, and what used to flow smoothly becomes lumpy. That means batch inconsistencies or—worse—equipment jams down the line. So, the best place for this material stays indoors, in a cool and dry place, out of direct sunlight. Keeping temperatures stable, ideally under 30°C, gives the product a much longer shelf life. Once, during a summer heatwave, storage over 35°C left the antioxidant less effective. No one needs to repeat that mistake.
Containers must seal up tight to block air and water from getting in. Most suppliers use steel or high-density polyethylene drums, so keep those lids shut. Double-check those seals after every use. An open lid, even for a short time, lets the ambient moisture creep into the material. Extra caution pays off when storms roll through, because even brief spikes in humidity will speed up degradation.
Unlabeled containers bring trouble. Even experienced teams can mix up different additives, with expensive results. Use clear, durable labels that won’t come off with a little condensation. Good organization, like separate racks for different chemicals, keeps cross-contamination away.
4,4'-Thiobis(6-Tert-Butyl-M-Cresol) gives off minimal odor, but it’s smart to avoid direct skin contact. Every workplace should make gloves, goggles, and long-sleeve shirts part of handling protocols. A friend of mine got careless and ended up with a rash—just because something isn’t corrosive, doesn’t mean it’s harmless over time. Wash hands after handling, and never store chemicals near food or drink.
Cleanliness beats cure every time. If any spills happen, sweep up the powder with a shovel or dustpan and wipe the area down. Don’t use water if you can help it; getting the product wet speeds the breakdown even faster. Store waste and clean-up materials in marked containers, and get rid of them following local rules.
Chemicals don’t last forever. Write down the delivery date and keep an eye on the oldest batches. Using the oldest stock first keeps the inventory fresh. If the product changes color or starts clumping, it’s probably time to test for purity. I’ve seen skilled operators spot problems by touch and appearance even before lab testing proves any drop in quality. Trust experience, but verify with periodic lab work.
More facilities are switching to automated environmental controls, which log temperature and humidity without extra paperwork. Simple investments like sealed, custom-built rooms or upgraded storage racks offer extra protection. Leaning into staff training, keeping everyone up to date on material safety data sheets, and sharing lessons from real-world mistakes all help dodge more serious accidents or supply chain downtime.
Safe storage stands as more than just a policy—it keeps high-value chemicals reliable and your team safe from unexpected trouble.4,4'-Thiobis(6-Tert-Butyl-M-Cresol) often ends up in labs, factories, and sometimes research facilities. It works well as an antioxidant in many industrial applications. The real problem starts once the job is done, and leftovers need discarding. Many chemicals go down the drain or into regular trash, but this isn’t some household waste. If not handled right, it could contaminate water, soil, and hurt anyone who comes in direct contact.
Some might ask, “What happens if I don’t dispose of this stuff carefully?” In the past, mishandling similar chemicals has led to health problems for workers, damaged local wildlife, and polluted our drinking water. I’ve seen former industrial zones in my own city where neglect stuck communities with toxic soil for generations. In one case, a small leak at a plant forced people to leave their homes for months so cleanup could happen safely.
Regulations like the Resource Conservation and Recovery Act in the U.S. mean companies must treat substances like this as hazardous waste. This isn’t just red tape. These rules exist because real people get sick if others cut corners. Even the dust from these chemicals can endanger lungs and skin, not to mention the havoc if the waste hits water supplies.
Good disposal always starts with a clear plan. My visits to chemical plants and university labs have taught me one clear lesson—workers need strong, simple safety procedures. Store this material in tightly sealed, labeled containers that can’t leak or break easily.
Bring in a licensed hazardous waste disposal service — someone trained in transport, treatment, and destruction of this kind of waste. These services use high-temperature incineration to break down the compound into harmless byproducts, all under well-controlled conditions. Dumping it down the sink or tossing in outdoor bins causes long-term headaches for everyone.
The Environmental Protection Agency and similar organizations in other countries offer clear directions for handing over industrial chemicals. Following their guide reduces risks dramatically, both for those working on site and for neighbors living nearby.
Disposal isn’t just about what to do after the fact. Thinking ahead helps too. Using only what’s needed and buying in smaller quantities keeps leftovers to a minimum. Some companies invest in closed systems—no leaks, nothing exposed, limited waste. On a recent tour, I saw a setup where process waste goes straight into sealed drums, picked up every week by a specialist contractor. Less risk, less drama.
Training staff makes a big difference. New lab members or hires may not know the dangers or the right steps. Simple posters and briefings help everyone stay on track. A culture of respect for both chemicals and coworkers cuts down on lazy habits.
There’s a certain peace of mind that comes with knowing no one’s at risk because of shortcuts. Whether you run a research bench, a factory floor, or a safety team, handling waste like 4,4'-Thiobis(6-Tert-Butyl-M-Cresol) with care protects the wider community and natural world. Taking those extra steps every time—labeling, safe storage, calling in professionals—builds trust and keeps future problems off the table.
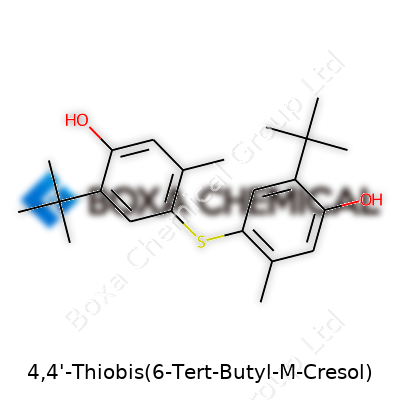
4,4'-Thiobis(6-Tert-Butyl-M-Cresol) doesn’t exactly roll off the tongue, but its structure reveals a lot about why manufacturers care about it. This antioxidant falls under the broader class of phenolic antioxidants. At the core, you get two cresol rings, which are methylated phenol groups, tied together with a sulfur (thio) bridge. Each cresol ring carries a bulky tert-butyl group, which brings stability to the molecule by shielding the reactive center from oxygen and free radicals.
The full chemical structure looks like this: each benzene ring holds a hydroxyl (–OH) group, a methyl (–CH3) group, and a large tert-butyl group (–C(CH3)3). The rings plug into opposite sides of a single sulfur atom, forming an elaborate but highly effective molecular shield. This arrangement allows it to trap harmful oxidative agents before they can damage other compounds, which helps protect plastics, rubber, and fuels that catch a lot of exposure to heat and air.
Through years of working in plastics manufacturing, one thing becomes clear: not all antioxidants are created equal. The chemistry shapes how an additive performs. The presence of two tert-butyl groups on each cresol ring in this molecule creates significant steric hindrance—a fancy way of saying the big groups crowd the ring. This crowding blocks oxygen and radicals from reaching the reactive hydroxyl group, enhancing lifespan and effectiveness.
The sulfur bridge contributes unique antioxidant capabilities. Sulfur tends to stabilize radical species, making the reaction with peroxides and other radicals more efficient. Compounds with similar sulfur linkages often show better resistance under thermal stress, which is crucial for products that get hot or face tough weather. That chemical toughness translates into longer-lasting polymers, less yellowing, and slower breakdown—all points that matter for both manufacturers and end-users.
Many industries turn to this antioxidant. Tires, automotive plastics, some adhesives, and lubricants owe much of their durability to additives like 4,4'-Thiobis(6-Tert-Butyl-M-Cresol). The chemical structure allows it to blend easily into many synthetic rubbers and plastics. Experience has shown that parts treated with this compound maintain shape, color, and elasticity longer than untreated items. If you’ve ever wondered why some dashboard panels crack in the sun and others survive for years, subtle touches like the right antioxidant can tip the scale.
A worry that crops up often involves environmental and health safety. Over time, persistent phenolic antioxidants like this one can leach out of plastics, potentially contaminating soil or water. Researchers track these molecules using sophisticated chromatography, searching for traces in the environment. The tert-butyl and sulfur groups slow down breakdown, increasing the potential for accumulation. Companies and regulators face a balancing act: preserving product durability while limiting environmental footprint.
Developers today investigate antioxidants that deliver the same robust protection but also degrade into harmless substances once their job ends. Some labs invest in tweaking the side groups or changing the bridge, aiming to preserve performance but speed up breakdown after use. Others look into recycling methods that capture antioxidants before plastic waste enters the ecosystem. Through ongoing testing and tighter regulation, the industry balances performance needs and safety, taking small steps toward a future where chemical additives do their work without leaving a lasting trace.
| Names | |
| Preferred IUPAC name | 4,4'-thiobis(2-tert-butyl-5-methylphenol) |
| Other names |
2,2’-Thiobis(4-methyl-6-tert-butylphenol) TBM-6 Antioxidant 300 6-tert-Butyl-4-methylphenol sulfide |
| Pronunciation | /ˈfɔːr fɔːr ˈθaɪ.oʊ.bɪs sɪks tɜrt ˈbɜːr.tɪl ɛm ˈkriː.sɒl/ |
| Identifiers | |
| CAS Number | 96-69-5 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4,4'-Thiobis(6-tert-butyl-m-cresol)**: ``` CC(C)(C)c1cc(C)c(O)cc1Sc1cc(C)c(O)cc1C(C)(C)C ``` |
| Beilstein Reference | 2059008 |
| ChEBI | CHEBI:135559 |
| ChEMBL | CHEMBL1401969 |
| ChemSpider | 91448 |
| DrugBank | DB14067 |
| ECHA InfoCard | echa.infoCard:100.020.387 |
| EC Number | EC 229-912-9 |
| Gmelin Reference | 58184 |
| KEGG | C11262 |
| MeSH | D008027 |
| PubChem CID | 66303 |
| RTECS number | GV3320000 |
| UNII | 3M1G07040A |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C28H42O2S |
| Molar mass | 410.65 g/mol |
| Appearance | white to light yellow powder |
| Odor | Odorless |
| Density | 1.08 g/cm³ |
| Solubility in water | insoluble |
| log P | 10.4 |
| Vapor pressure | <0.01 mmHg (25°C) |
| Acidity (pKa) | 11.87 |
| Basicity (pKb) | 11.4 |
| Magnetic susceptibility (χ) | -57.0e-6 cm³/mol |
| Refractive index (nD) | 1.100 |
| Dipole moment | 2.2 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 502.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -8355 kJ/mol |
| Pharmacology | |
| ATC code | A05BA02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. Suspected of causing cancer. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 3-1-1-W |
| Flash point | > 265°C |
| Autoignition temperature | 315°C |
| Lethal dose or concentration | LD50 oral rat 6200mg/kg |
| LD50 (median dose) | 2000 mg/kg (rat, oral) |
| NIOSH | NIOSH: WZ4725000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 130 mg/kg |
| Related compounds | |
| Related compounds |
2-Mercaptobenzimidazole 2-Mercaptobenzothiazole Thioanisole Thiophenol |