Tracing the story of 4-(2-Methoxyethyl)phenol feels like flipping through the forgotten pages of postwar chemical discovery. Aromatic alcohols attracted the attention of researchers as synthetic methods advanced in the 1950s and 1960s. Not every compound in the growing family grabbed headlines, but the introduction of flexible side chains onto phenolic backbones marked a shift. Chemists used trial and error to open fresh opportunities in dyes, pharmaceuticals, and polymers. The addition of a methoxyethyl group represented a nudge to regular phenol, creating just enough distance from the regular compound to discover differences in reactivity and application. It didn't sweep through industry overnight, but steady experimentation in research labs, coupled with globalization of chemical production, brought this molecule closer to factories and pharmaceutical pipelines as demand for specialized phenolic derivatives grew in the closing decades of the twentieth century.
Product catalogues list 4-(2-Methoxyethyl)phenol as a clear to pale yellow liquid or sometimes a low-melting solid, easy to store in sealed vessels. Its composition blends the robustness of phenolic core with the subtle solvent power from its alkoxy side chain. This combination provides compatibility in synthesis of advanced chemicals, plasticizers, and additives. I've seen it nestled among research samples destined for larger batch production, always demanding handling protocols matched to its moderate volatility and mild but persistent aroma.
This compound shows up with a molecular formula of C9H12O2 and a molecular weight of about 152.19 g/mol. It tends to display a melting point hovering around 25–30°C, with a boiling point usually cited close to 280°C at atmospheric pressure. Solubility in water registers as low, but organic solvents like ethanol, ether, and chloroform dissolve it with little resistance. The addition of the methoxyethyl substituent tunes its polarity, sitting between the reactivity of simpler phenols and the greater hydrophilicity of more heavily functionalized ethers. These tweaks affect how it interacts in condensation reactions or as a solvent component. In my own chemical storage, comparable substances have demanded clear labeling and humidity control to protect against slow oxidation.
Regulatory bodies expect tight control over labeling. Batches must carry information including CAS number, purity grade, batch ID, and known impure markers. Purity levels can stretch above 98%, aimed at analytical or pharmaceutical uses. Impurity profiling often includes related ethers, residual solvents, and trace synthetic byproducts. Technical sheets from reliable suppliers lay out flash points, storage guidelines, shelf life expectations, and recommended personal protective equipment. In my work, overlooking any aspect of product documentation spells disaster, especially given the compound's moderate toxicity and flammability.
Synthesis taps into established phenol chemistry, utilizing etherification to attach the methoxyethyl chain at the para position. A common approach starts from hydroquinone, selectively alkylating with 2-methoxyethyl halides in presence of strong base or phase-transfer catalysts. Reaction temperatures and times demand careful tuning, as over-alkylation or isomer formation can crowd the output with side products. Lab-scale preparation involves distillation under reduced pressure to isolate the pure compound. Large-scale plants introduce greater automation and solvent recovery, cutting energy usage by recycling heat and waste streams. My years in pilot production have demonstrated the real benefit of tracking yields and recycling solvents wherever practical, both for cost control and environmental responsibility.
The molecule's phenolic hydroxyl remains mildly reactive, allowing further transformations such as esterification, etherification, or even Fries rearrangements. Electrophilic aromatic substitution still proceeds under strict conditions, but the side chain modulates both reactivity and regioselectivity. The methoxyethyl moiety preserves the parent ring’s nucleophilic and radical reactivity, enabling cross-coupling with halides and participation in Mitsunobu reactions. Greeks and cross-coupling fans in modern research circles appreciate the balance achieved in this structure, leveraging it as a building block rather than a finished product. This kind of flexibility and the option for further derivatization keep it circulating on wish lists for medicinal and fine chemical synthesis.
4-(2-Methoxyethyl)phenol turns up in databases and purchasing portals under names like p-(2-Methoxyethyl)phenol, 4-(2-methoxyethyl)hydroxybenzene, and even 4-Hydroxyphenethyl methyl ether. International suppliers sometimes adopt abbreviated trade names or catalog codes, but every reputable label pinpoints the compound through substance identification and structure. R&D chemists note the importance of cross-checking synonyms to avoid confusion, particularly when assessing literature or regulatory data for safety and performance profiles.
Handling protocols for 4-(2-Methoxyethyl)phenol owe much to its moderate toxicity and skin sensitivity. Workers must wear chemical-resistant gloves, splash goggles, and utilize fume hoods to minimize inhalation risks. Material Safety Data Sheets flag hazards including eye injury and respiratory irritation, and regular ventilation checks help prevent any buildup of vapor. Absorbed through skin or inhaled as aerosol, the substance triggers irritation and, at higher doses, systemic effects. Real-life laboratory practice stresses the importance of sealed containers kept away from oxidizers or strong acids, and every incident of improper handling highlights the need for clear labeling and regular hazard communication. Training new staff on safe handling routines often relies on such case studies, establishing habits that persist across a career.
The applications for this phenolic ether stretch across chemical synthesis, specialty polymers, and pharmaceutical intermediates. Chemical industries use it as an intermediate in crafting more elaborate phenolic resins and plastic additives that demand precise thermal and mechanical properties. I’ve seen it show up in trial batches of adhesive modifiers, blending durability with improved processing behavior thanks to the flexible alkoxy side chain. Pharmaceutical labs identify it as a building block for drugs targeting central nervous system and anti-inflammatory pathways, banking on the improved membrane penetration that comes with its ether group. Fine-chemical producers add it to their roster for coupling reactions, color stabilizers, and bioactive compound precursors. My contacts in polymer science note its utility in niche resin formulations, boosting compatibility or imparting unique curing properties to emerging materials.
Innovation around aromatic ethers means research groups keep tinkering with the synthesis and applications of 4-(2-Methoxyethyl)phenol. Studies target greener synthesis—lowering solvent use and designing catalysis that reduces waste. Labs publish efforts to streamline preparation, improve atom economy, and cut energy demands. New coupling strategies reduce hazardous byproducts, and analysis of reaction mechanisms fills journals with insights that feed industrial scale-up. Discovery of structure–activity relationships broadens its pharmaceutical application, with researchers probing how side-chain modification affects drug penetration and metabolic pathways. After long hours with data, many chemists recognize the subtle tradeoffs between reactivity, toxicity, and process cost—it’s an ever-shifting puzzle.
Toxicology investigations report that 4-(2-Methoxyethyl)phenol poses skin and eye irritation risks, with higher exposure resulting in systemic effects such as liver and kidney stress in animal models. Acute toxicity studies reveal moderate oral and dermal LD50 values, characterizing it as more dangerous than simpler ethers but less potent than halogenated phenols. Chronic exposure data remains sparse, pushing researchers to seek more nuance around biotransformation and residue accumulation. Medical researchers responsible for occupational safety echo a need for detailed exposure monitoring and periodic health checks, especially for factory operators dealing with regular contact. Data so far suggests the compound does not bioaccumulate at alarming rates; nonetheless, vigilance remains the rule. Environmental toxicologists warn against even low-level release into waterways, as the breakdown products display moderate aquatic toxicity.
Looking ahead, the experience of chemists, engineers, and product developers points to more sustainable synthesis, tighter product purity, and new reaction pathways. Companies in North America, Europe, and East Asia already invest in optimizing the production lines, adopting continuous-flow synthesis for better scalability and improved worker safety. Researchers study how subtle modifications at the ether side chain create not only incremental improvements but also unlock entirely new families of active agents and materials. Green chemistry remains at the forefront—biocatalysis, solvent-free procedures, and recyclable supports frame how this compound advances in the next generation of manufacturing. Regulatory requirements grow stricter over time, making thorough documentation and real-time monitoring an industry standard. All signs point to a future where transparent supply chains, evidence-driven safety measures, and design-for-environment thinking drive innovation for compounds just like 4-(2-Methoxyethyl)phenol.
4-(2-Methoxyethyl)phenol might not ring a bell for many, but this compound finds itself at the crossroads of chemistry and industry. In my lab days, the search for new building blocks for synthesis often led back to molecules like this. Chemists rely on straightforward phenolic compounds to channel reactivity in the right direction. This one, in particular, keeps showing up in three main sectors: pharmaceutical development, specialty polymer production, and fragrance formulations.
Drug discovery doesn’t always look for big, dramatic structures. Sometimes the answer lies in a simple tweak. The presence of both a phenolic group and a methoxyethyl chain gives 4-(2-Methoxyethyl)phenol a profile favoring modification. Medicinal chemists appreciate it as an intermediate in the search for painkillers, anti-inflammatory drugs, and even antimicrobials. The structure lets researchers attach new branches easily, shaping molecules that block pain or slow the spread of bacteria. Studies have shown substituted phenols play a role in antioxidant activity, giving hope for new therapies targeting oxidative stress. There’s a direct connection between the flexibility of this backbone and breakthroughs at the lab bench.
The plastics we handle daily have history. High-performance polymers count on aromatic compounds like this one, especially for custom tailoring. Manufacturers hunt for specialty monomers that combine strength, flexibility, and resistance to cracking. 4-(2-Methoxyethyl)phenol’s balance of polar and nonpolar features gives it an edge in synthesizing resins and polyesters that need to resist corrosion or heat. End uses pop up in coatings, adhesives, and occasionally in molded consumer goods. It strikes a middle ground—not as soft as rubber, but not nearly as brittle as pure polystyrene.
I remember walking into a fragrance lab for the first time and being surprised by how much chemistry happened behind the scenes. Perfumers look for aroma molecules with staying power and subtle, complex backgrounds. 4-(2-Methoxyethyl)phenol offers exactly that, combining a sweet, ether-like note with woody hints. Its stability means lasting impact in perfumes and deodorants, especially those marketed for endurance. Some research has gone into using this material for odor-masking too, since the methoxyethyl group can tamp down harsher phenolic scents. It slides into formulations for air fresheners and functional fragrances—places where science and smell overlap.
Compounds like this always come under scrutiny, which feels necessary and wise. Long-term exposure considerations jump out, since phenolic chemicals sometimes irritate skin and sensitive cells. Responsible manufacturers test for toxicity and corrosion constantly—a practice that ought to stay front and center. Finding greener, biodegradable options for future applications might shift attention, but at present, the blend of reactivity and functionality keeps 4-(2-Methoxyethyl)phenol in play.
It’s worth backing research into alternatives and stricter oversight. I’ve seen growing demand among startups for bio-based versions of traditional monomers and intermediates. Partnerships between chemists, toxicologists, and industrial designers will help uncover better options. For now, the main uses of 4-(2-Methoxyethyl)phenol showcase both the ingenuity and the challenges of developing smarter, safer chemicals for real-world needs.
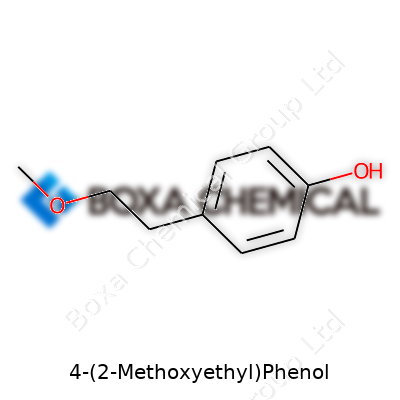
Anyone who’s ever set foot in a chemistry lab gets that every molecule brings its own story. If you’re curious about what makes 4-(2-Methoxyethyl)phenol unique, it starts at its core — a benzene ring. This six-carbon ring forms the backbone, creating an aromatic base that’s found in tons of everyday substances. Off the fourth spot of that ring, chemists have attached a 2-methoxyethyl group. On the other end, at the first position, a hydroxyl (-OH) group tags along. Put together, this means you’re looking at a benzene ring with two groups sticking out: one is the -OH group (which turns the molecule into a phenol), and the other is a chain —CH2CH2OCH3 — that brings in the methoxyethyl twist.
The phenol group punches above its weight. That -OH group adds acidity, which means the molecule can interact with other chemicals in ways pure benzene never could. This also boosts its solubility in water, which sometimes makes handling in the lab much neater. Shift your gaze to the 2-methoxyethyl side chain — this adds flexibility, literally. Chains like this bend and stretch, letting the molecule slip into roles you wouldn’t expect from the stiff and flat benzene ring alone. The ether oxygen in the methoxy part (-OCH3) is a known player in pharmaceuticals, paints, and surfactants for the way it tweaks reactivity and boosts solvent power.
Years of working with phenol derivatives taught me that these tweaks in chemical structure make a difference. Small structural changes can shift a compound from being an environmental hazard to a pharmacy shelf staple. The electron-rich ring, topped with a hydroxyl group, often shows up in antioxidant agents, polymers, and flavors. With the 2-methoxyethyl group onboard, you’re adding both reactivity and the potential for custom chemical synthesis. That side chain can temper the molecule’s inherent reactivity, opening the door for applications where you want the benefits of phenols without their strong bite. In the lab, these molecules usually dissolve easily in many solvents, which brings straightforward mixing and processing — something anyone running reactions by hand will appreciate.
Phenols always deserve respect. Their strong biological activity means they can protect or harm. They often pop up in preservatives, but may also irritate skin or disrupt marine ecosystems if poured down a drain. Using phenolic compounds, especially with additional groups like methoxyethyl that improve cell penetration, can amplify both their effectiveness and their toxicity. Responsible handling is non-negotiable, and personal protective gear wasn’t made just for show. I’ve seen students underestimate phenols before — the lesson sticks after even a minor chemical burn. The right ventilation, gloves, and properly labeled containers make up an essential first line of defense.
Finding safer ways to work with and dispose of structure-modified phenols should rank high for labs and industry. Decades ago, these compounds often went straight into waste streams; nobody really thought about the environmental punch they packed. Now, closed-loop systems and on-site chemical treatment help cut down exposure. Exploring ways to engineer molecules with the same utility but less environmental baggage could push the field forward. Green chemistry isn’t just a buzzword — it’s how we keep making lifesaving drugs and reliable materials without throwing more hazards into the mix.
Many people see chemistry as something that happens in distant factories or university labs. In truth, chemicals like 4-(2-Methoxyethyl)Phenol show up in research, small companies, and even custom manufacturing jobs. This compound doesn’t make headlines, but mishandling it leads to big safety issues and real costs. I still remember standing in a shared academic prep room, catching the sharp, medicinal smell from a mislabeled bottle. That day we spent hours ventilating the space and hunting down spilled droplets. Someone skipped basic safety, and that created chaos nobody needed.
Temperature swings ruin a whole bottle. Storing this compound in a cool, dry area avoids problems—moisture damages purity and warmth increases risks of unwanted reactions. Any staff member can check the thermometer and recognize the signs of dampness. A locked cabinet, away from sunlight and heat, seems low-tech but blocks half the issues before they start.
Leaks never announce themselves. Clean glass containers with tightly fitting lids work well. Old bottles with cracks or those thin, flexible plastics turn into accidents waiting to happen. Labeling skips confusion and prevents someone from mixing or mishandling contents. I learned early on that poorly labeled or repurposed bottles almost always end up in safety incident reports. Hazard symbols and clear names matter most during emergencies or fast-paced work.
Many forget that fumes matter as much as spills. Working in well-ventilated rooms makes a difference. I’ve watched someone in a closed storeroom struggle because vapors had nowhere to go. The solution sits in basic ventilation fans and good airflow design. Open a window, install a vent hood, and monitor for chemical odors often. This kind of proactive approach saves both health and time down the line.
Some folks think gloves and goggles belong in lab demonstrations. I’ve seen chemical burns from a moment’s distraction. Keeping gloves, eye protection, and chemical aprons within arm’s reach removes excuses. The right routine starts with habit, with workers seeing these tools as everyday gear—not something reserved for worst-case scenarios.
No one loves chemical inventories. Skipping them only piles up mystery bottles with faded labels. Every bottle should carry an expiry date and a record of opening. Tracking these details keeps surprises off the shelves. Unused or expired 4-(2-Methoxyethyl)Phenol needs prompt, regulated disposal. Many places restrict pouring chemicals down the drain, which protects both workspaces and local water supplies. Certified waste pickups clear out old stock without risk. Up-to-date safety data sheets help everyone make smart choices.
In my experience, effective chemical storage grows from shared responsibility. Training sessions and clear rules encourage everyone to speak up when something looks wrong. Drills, real-life stories, or peer checks cut down on shortcuts and “good enough” behaviors. The best facilities I’ve worked at keep chemical storage routines simple but never take safety for granted.
4-(2-Methoxyethyl)Phenol catches attention not just for its complex name. This compound, often found in labs and chemical supply rooms, can irritate skin and eyes quickly. Take a heavy whiff by mistake, and you’ll notice its impact on your lungs. Science groups like OSHA and NIOSH list phenolic compounds as substances worth treating carefully. Even a short slip becomes risky if a splash or spill happens in a busy lab.
In my early days working chemical prep, some folks wore only thin gloves because hot weather made layering up tough. Someone always ended up with rashes or stinging fingers. Over time, we learned extra layers weren’t just for cold days. Thick nitrile gloves, safety goggles covering all sides, and sturdy lab coats help block this phenol from skin and eyes. Standard glasses leave gaps—goggles matter. Fume hoods play a big role since this chemical can evaporate. I learned from a lab neighbor who once mixed phenol near an open window without proper venting. The aftereffects lingered for hours.
It sounds obvious but working in a shared lab tempts people to eat or drink at their bench. Any hint of 4-(2-Methoxyethyl)Phenol spills multiplies that risk. Food absorbs vapors, surfaces retain residues, and soon someone wonders why their sandwich tastes odd. People make mistakes if they rush. Measuring carefully, working thoughtfully, and double-checking bottle labels lower the odds of getting the wrong liquid in a pipette or flask.
Every spill tells a story, especially with tricky chemicals. I’ve seen towels tossed in the trash only to realize hours later the odor still filled the room. Proper cleanup means using special absorbents and gloves, then sealing waste in containers marked with clear warnings. Local disposal rules need checking, since phenolic wastes demand extra care before heading out of the building.
Labs that invest in spill kits and post emergency numbers see fewer panicked searches for help after an accident. Drench hoses and eyewash stations save seconds; I once saw a splash turn minor because a quick rinse happened within moments. Without easy access, someone could spend crucial minutes running down hallways, which makes injuries worse.
Reading instructions once and calling it good rarely works. Refresher sessions, signage, and hands-on drills build good habits. A team that stays curious about risks avoids messy outcomes. I watched as a new teammate skipped a glove change after a spill; speaking up right then likely kept their hand from burning. Colleagues who support each other catch small mistakes before they become real emergencies.
Given the risks, safe handling of 4-(2-Methoxyethyl)Phenol starts with solid habits and honest teamwork. It’s not about fear—it’s about coming home healthy at the end of each day. With the right protection, cleanup, and training, using this potent chemical can happen without trouble.
A simple bottle of 4-(2-methoxyethyl)phenol on a laboratory shelf might look unassuming, but what lies within can make or break a synthesis. In production labs, chemists rarely take a supplier’s label at face value. Instead, the focus always shifts to reliability—especially in pharmaceuticals, specialty polymers, and advanced coatings, where the smallest impurities can alter chemical behavior, hurt yields, or even compromise safety.
Over the years, several experiences have underscored that minor variances in purity can spark headaches. Research demands batches that closely match one another. Pharmaceutical labs often specify purity upwards of 98%, usually on a dry basis, with analytical methods such as high-performance liquid chromatography (HPLC) or gas chromatography (GC) as documented proof.
Suppliers in Europe and North America tend to keep their minimum purity standards for 4-(2-methoxyethyl)phenol at or above 98%. Analytical certificates routinely list content at 98.0–99.5%, with water content below 0.5%, and low levels of heavy metals or related aromatic impurities. Side-products, like unreacted precursors or substituted phenols, get restricted to less than 0.2%. For work involving catalysts or custom material synthesis, even these trace levels might cause issues or forced reworks.
Labs in academic settings might tolerate a larger impurity profile, aiming for cost savings or where downstream purification is built into the protocol. Commercial production cannot afford this luxury. Batch-to-batch consistency trumps theoretical maximums—any shift in purity, even within specification, will change the yield, purity, odor, even the color of a downstream product. Chemical traceability and transparent Certificates of Analysis fill out the picture and build trust in the supply chain. I’ve seen researchers reject entire shipments because HPLC signatures didn’t match their required threshold, even if a paper certificate claimed compliance.
It’s never quite enough to assume pure means fit for purpose. Smart teams request analytical data before purchase and use tools like nuclear magnetic resonance (NMR), FTIR, and mass spectrometry to check for residual solvent, trace oxidants, or tars. Purification can boost specifications—simple distillation or recrystallization often strips off low-level contaminants. Some pharma customers require documentation for every stage: isolation, drying, and packaging, to combat hidden variables.
The supply chain remains the most unpredictable factor. During material shortages, suppliers sometimes relax internal controls, pushing ‘off-grade’ material into regular channels. In these periods, hands-on verification—beyond the certificate—proves vital. Any chemist who’s been burned by a failed reaction because a bottle of “>99%” actually contained 0.7% unreported phenolic analogues learns to double-check, especially on projects with little margin for error.
Above all, consumer safety drives companies to specify, chase, and document purity. Drug ingredients, or chemicals finding their way into food packaging, demand almost surgical precision. Even tiny impurities can impact human health or regulatory compliance. Without ironclad traceability and honest reporting, these risks become invisible until too late. So a figure like “purity ≥98%” never acts as a brag—it’s part of a chain of accountability, stretching from lab benches to the world beyond.
| Names | |
| Preferred IUPAC name | 4-(2-Methoxyethyl)phenol |
| Other names |
Phenol, 4-(2-methoxyethyl)- 4-(2-Methoxyethyl)phenol 4-Hydroxyphenethyl methyl ether 2-(4-Hydroxyphenyl)ethyl methyl ether |
| Pronunciation | /ˈfɔːr tuː ˌmɛθ.ɒk.siˈiːθɪl ˈfiː.nɒl/ |
| Identifiers | |
| CAS Number | 6138-44-3 |
| 3D model (JSmol) | `3DModel:JSmol:Cc1ccc(O)cc1CCOC` |
| Beilstein Reference | 84264 |
| ChEBI | CHEBI:89872 |
| ChEMBL | CHEMBL285002 |
| ChemSpider | 171427 |
| DrugBank | DB08355 |
| ECHA InfoCard | 100.175.167 |
| EC Number | 624-880-3 |
| Gmelin Reference | 175115 |
| KEGG | C19596 |
| MeSH | D029420 |
| PubChem CID | 11665797 |
| RTECS number | SK7175000 |
| UNII | 00R5F6A8G9 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4021533 |
| Properties | |
| Chemical formula | C9H12O2 |
| Molar mass | 152.19 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | pleasant, sweet |
| Density | 1.108 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.41 |
| Vapor pressure | 0.0426 mmHg (25°C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 7.15 |
| Magnetic susceptibility (χ) | -47.4·10^-6 cm^3/mol |
| Refractive index (nD) | 1.553 |
| Viscosity | 2.4 cP (20°C) |
| Dipole moment | 2.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -274.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3349.8 kJ/mol |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | H315, H319, H335 |
| Flash point | 121°C |
| Autoignition temperature | 300°C |
| Lethal dose or concentration | LD₅₀ (Oral, Rat): 835 mg/kg |
| LD50 (median dose) | LD50 (median dose): 631 mg/kg (rat, oral) |
| NIOSH | PJ5775000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 ppm (6 mg/m³) |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Phenol 4-Ethylphenol Guaiacol 4-(2-Hydroxyethyl)phenol 4-Methoxyphenol |