The journey of 3-Nitrophenol traces back to the late 19th century, a period marked by remarkable advances in organic chemistry. Back then, scientists sought to understand the structure of aromatic compounds and their functional groups. This molecule, with its nitro group snug at the meta-position on the phenol ring, presented unique reactivity. Early studies didn’t just spark interest in its preparation—they also seeded questions about its behavior under various conditions. My fascination with its origins grows every time I see how foundational these early experiments were for both basic academic research and industrial chemistry. Real progress started as researchers mapped out methods like direct nitration of phenol, eventually opening doors for countless downstream uses.
3-Nitrophenol stands as a yellow crystalline solid used in laboratories, chemical synthesis, and in the production of specialty dyes and pesticides. Its role extends to serving as an intermediate in pharmaceutical production and as a precursor for more complex molecules. One of the things I find compelling is its reactivity, which brings flexibility for chemical manufacturers. Formulators and researchers alike value it for the distinct properties the nitro group imparts to the aromatic ring, not just as a building block but as a target for exploring new molecular landscapes in synthesis.
This compound comes with a melting point of around 97°C, boiling up near 279°C where some decomposition can set in. Its moderate solubility in water gives it just enough mobility to make lab work straightforward, without posing the hazards of highly mobile toxins. Its molecular formula is C6H5NO3, tipping the scales at about 139.11 g/mol. The nitro group doesn’t just alter electron density on the ring—it impacts colors, UV absorbance, and reactivity. Years in labs have shown me how the meta-nitro placement differs from ortho and para variants both in handling and in reactivity. This version holds its ground in acidic and neutral conditions but can break down under strong reducing agents.
Higher-purity batches of 3-Nitrophenol are critical for research purposes, with purity checks handled using chromatography, melting point, and spectral data. Each container ought to carry clear hazard symbols, emphasizing acute toxicity and environmental risks. Accurate batch labeling, CAS registration, and regulatory compliance information matter not just for legal reasons—safety in storage and use depend on everyone knowing exactly what they’re dealing with. In my experience, attention to labeling details avoids mix-ups and potentially dangerous errors, especially in busy multi-use labs. Keeping up with regulatory bodies also means adjustments in transport and disposal practices to meet evolving standards.
Most labs generate 3-Nitrophenol by nitrating phenol with diluted nitric acid in the presence of sulfuric acid. Conditions get tightly controlled to avoid over-nitration or unwanted isomers. Reaction temperature, acid ratios, and mixing speed all influence yield—a lesson anyone who’s ever run this synthesis learns quickly. Filtration, washing, and recrystallization refine the product, stripping away colored impurities and unreacted starting materials. Scaling this up from the bench to industrial reactors introduced challenges—heat control, waste management, and worker safety all come sharply into focus.
Chemists treasure 3-Nitrophenol for the range of transformations it supports. Reduction produces 3-aminophenol, a useful dye precursor. The nitro group’s electron-withdrawing effect alters the ring’s reactivity, supporting substitutions not always possible in the parent phenol. Halogenation often goes smoothly, and alkylation leads in multiple directions for custom synthesis. Many nights in undergrad labs showed me just how the meta-position of that nitro group can open or block certain routes—a fact as important in textbooks as it is in real-world process development. Coupling reactions, such as those forming azo dyes or linking aromatic blocks, benefit from the predictable chemistry of this structure.
Industry documentation lists synonyms like m-Nitrophenol, Meta-nitrophenol, and 3-Hydroxynitrobenzene. CAS number 554-84-7 identifies it clearly across global markets. On bottle labels and product sheets, names sometimes reflect company-specific codes, but core chemical names and registry numbers matter more for correct identification and supply-chain traceability. Communicating across international borders, I've noticed how consistency in these naming conventions reduces errors and speeds up procurement for time-sensitive projects.
Exposure to 3-Nitrophenol requires care. Direct skin contact or inhalation carries acute health risks. Lab protocols demand gloves, eye protection, and sometimes fume hood use—rightly so, given its toxicity. Regulatory frameworks such as OSHA and REACH set strict limits and reporting requirements. Spill handling and waste management plans must be in place before opening even a single container. My time working safety committees made clear that routine practice softens vigilance—the real discipline comes from staying current with updated protocols and investing in staff training, especially for teams newer to hazardous organics.
Aromatic nitro compounds like 3-Nitrophenol show up in chemical manufacturing, dye production, drug synthesis, and, in smaller volumes, in advanced material research. In the dye industry, it produces vivid shades through coupling with amines and other aromatic systems, making it a linchpin molecule for textile chemistry. In pharmaceuticals, it works as a building block in multi-step syntheses. Over the years, I’ve encountered it in pigment production and photographic developer research, where subtle shifts in structure produced big changes in end-use properties. Environmental chemists have also studied it to track nitroaromatic pollutant pathways—a vivid reminder that chemical value brings responsibility too.
Recent research scrutinizes greener synthesis methods for 3-Nitrophenol to reduce by-products and hazardous waste, with some progress in using milder nitrating agents and catalysis. Material scientists consider new derivatives for polymer chemistry, particularly as possible cross-linking agents or specialty additives. Pharmaceuticals keep returning to the phenol ring, probing how substitution patterns affect biological activity. Part of what keeps lab work exciting is the ongoing search for novel transformations—each tweak in method or functional group brings different biological, optical, and physical outcomes. Funding drives for innovation now emphasize sustainability, greener solvents, and process intensification.
Animal studies point to acute and chronic health risks, with effects on the liver, kidneys, and blood. Even short-term exposure increases health concerns, pushing for lower workplace limits and special handling rules. Ecotoxicity reports show threats to aquatic life, making discharge into water systems unacceptable under most regulatory codes. My own review of industrial hygiene reports forces respect for controls; even robust containment and ventilation can’t substitute for proper training and readiness to address accidental leaks or exposures. Public health agencies now include 3-Nitrophenol in environmental monitoring for contaminated sites and hazardous waste studies, again underscoring a tension between scientific progress and safety needs.
Coming years may see shifts in demand as sustainability shapes priorities in chemical production, synthesis, and regulatory focus. Bio-based production methods, such as engineered bacterial consortia or catalyst-driven green synthesis, spark investor and academic curiosity. Downstream, modifications of the aromatic core could yield new materials with optical or electronic functions. Working in R&D, I often see how old molecules get fresh attention—3-Nitrophenol remains a reference point for innovation. Balancing commercial interest with safety and environmental care will challenge both researchers and industry, especially as legislatures act on emerging science about workplace and ecosystem effects. The story of this substance keeps growing, showing how each generation of chemists adds nuance to the science and stewardship around legacy chemicals.
3-Nitrophenol forms part of a group of chemical compounds that have shaped both scientific research and industry. Anyone who has spent time in a chemistry lab knows the pungent, almost medicinal smell that lets you know this molecule – or something like it – is close at hand. While the name sounds technical, the ways this compound affects daily life aren’t always obvious. Let’s break it down.
From an early stage, industries have relied on 3-Nitrophenol for its role in making dyes and pigments. Textile plants and ink manufacturers pick this compound for its color-giving abilities. Its chemical structure helps create bright, stable shades. If you’ve ever washed a piece of clothing and noticed it still holds its color after many cycles, you’ve seen the end result of years of chemical design, and 3-Nitrophenol has often played a hand in that process.
Farmers and agricultural researchers know chemicals can make or break a season’s yield. 3-Nitrophenol gets used as an intermediate in creating pesticides and herbicides. Its presence in these products lets growers fight back against weeds and bugs without risking crops themselves. Environmental agencies keep a close eye on the use of such substances, as run-off and misuse can hurt surrounding plant and animal life. The challenge: keep food production up, but never lose sight of health and safety.
Drug creation often demands reliable intermediates. 3-Nitrophenol pops up in labs developing medicines for both people and animals. It acts as a building block, helping chemists craft products that relieve pain, lower fevers, or treat infections. In my time working with research teams, breakthroughs often depended on the right starting materials. One misstep could delay progress. Using trusted compounds like 3-Nitrophenol made complex projects possible and kept them on track.
Environmental labs have another use for this compound. In some tests, 3-Nitrophenol works as a standard or a marker for detecting pollutants in water and soil. With urban expansion and industrialization, pollution monitoring grows more vital by the year. Tools and chemicals that reveal even tiny traces of contamination help communities tackle health risks early.
With every benefit, questions about harm follow. Toxicity of 3-Nitrophenol has attracted plenty of scrutiny. Lab staff need to keep their gloves on, and factory managers set strict safety protocols. Handling and disposal count for everything. Organizations such as the Environmental Protection Agency and the European Chemicals Agency keep reviewing data and issue guidelines to limit risks. Scientists have started exploring greener chemistry methods, searching for safer alternatives or ways to recycle and detoxify waste from manufacturing processes.
It’s easy to overlook the chemicals behind familiar goods – medicines, dyes, or crop treatments. 3-Nitrophenol isn’t exactly a household word, but its reach extends into mainstream products. Being honest about its pros and cons lets everyone take smarter steps – whether working the lab bench or farming the land. Building a balance between progress and protection won’t end soon, but people who work with these compounds know safety and responsibility always come first.
Getting near 3-nitrophenol or working with it in labs isn’t a job to take lightly. This yellow, crystalline solid sees use in making dyes, drugs, and some rubber chemicals—but its hazards feel real once you read the safety data sheet. Your skin, eyes, and lungs don’t get along with it. Even a little bit can irritate, and enough exposure spells trouble for blood, kidneys, and the nervous system. Transport and spills pose their own problems. So common sense and trusted guidelines deserve respect every time someone handles this stuff.
Every person who works with 3-nitrophenol needs gloves—and not the cheap, thin kind. Nitrile or neoprene gloves give a strong barrier. Eye protection such as snug goggles or plastic face shields belong on your head whenever bottles get opened. The dust likes to drift, so most labs and workspaces call for a properly fitted respirator (like N95 or above) if there’s dust or vapors in the air.
Windows don’t cut it. Hoods with real airflow help keep airborne 3-nitrophenol away from lungs. Anyone who works around chemicals picks up stories—one missed vent check, one turned-off fan, and someone gets coughing fits or worse. So, make sure the exhaust system pulls fumes out, not just recycles air back into the building.
Every science teacher carries memories of someone accidentally brushing a sleeve against a beaker, or smuggling a bit of trouble home on their sweater. 3-nitrophenol calls for buttoned lab coats, no loose sleeves, and thorough hand washing before you eat, drink, or leave. Stash snacks and drinks away from benches to keep residues out of lunch.
Everyone makes mistakes, but chemicals rarely forgive them. A sealed, clearly labeled container belongs in a cool, dry spot—no metals around to spark a reaction. Separate acids, bases, and oxidizers, since mixing them with 3-nitrophenol spells out danger. Spills need more than paper towels; absorbents designed for acids handle the mess better, and contaminated items belong in dedicated hazard bins.
Fast action matters. People who skip drills or emergency plans often freeze up during a spill. Map out exits, keep dustpans, neutralizing agents, or spill socks nearby, and walk through the steps out loud with your crew now and then. If skin or eyes make contact, rinse with clean water—not for a second, but for at least fifteen minutes. Don’t hesitate to call poison control or emergency responders.
Pouring this stuff down any drain leads to headaches for water treatment and big bills for cleanups. Companies and schools should reach out to licensed hazardous waste handlers for pick-up. Track each disposal, check local rules, and hold on to proof—regulators don’t care about “I thought it was fine.”
Reading the manual works—but not as well as actually quizzing each other or running real-life practice. OSHA and other agencies publish practical advice that helps keep everyone accountable. In my time around college labs, the folks who read safety rules aloud and made time for drills surprised fewer coworkers with accidents.
Engineers keep looking for substitutes that bring the same results without so many risks. Until then, staying sharp around 3-nitrophenol pays off every single shift. Safety gear, habits, cleanup plans, and training give people—and the planet—a better shot at staying healthy.
Working with any chemical calls for some real respect. 3-Nitrophenol deserves that kind of attention for good reason—this yellowish compound isn’t as innocent as it looks. I’ve seen enough stories about chemical mishaps in research and industry labs to know that skipping proper storage quickly turns into a safety issue. The stuff can cause irritation or much worse. The Environmental Protection Agency puts this compound on a list of potentially hazardous substances, and health agencies point out risks ranging from skin and respiratory irritation to environmental harm. So anyone who winds up responsible for it owes it to themselves and everyone around to treat it with care.
I learned early on in lab work that some chemical containers do a lousy job against permeation or reaction. Not all plastics hold up, especially with phenolic compounds like this. Glass containers with tightly sealed lids do the trick. Polyethylene seems to play along too, but I’d leave it to glass if there’s any doubt. Lids should always close snugly—moisture and air are no friends here, and exposure can encourage degradation. Any time I noticed bottles that didn’t close right, I replaced them without a second thought.
Lighting and temperature change the game for 3-nitrophenol. Direct sunlight and fluorescent lights speed up chemical breakdown. Shelving in a dark storage cabinet, preferably metal, steps in to control stray light. I always double-checked for temperature swings, since this compound does best in cool, stable climates. A spot somewhere between 15 and 30°C keeps the risk low. Facilities with climate control take an edge, but if I was in a place without that, I checked on storage every day—opening a sticky, crystallized bottle can be a rude surprise.
Mixing up storage leads to chaotic results—3-nitrophenol should never sit near acids, strong oxidizers, or reducing agents. From my experience, the best policy involves proper segregation, and clear signage in the storage area. More than once I caught people swapping labels or putting things back in the wrong spot. That’s how accidents start. Installing secondary containment, like a spill tray underneath bottles, saves time and cleanup headaches if a mess happens. I’ve seen this investment pay for itself by stopping leaks before they turned into lab shutdowns.
Training does wonders. Newcomers sometimes miss hazards, so refreshers help people spot trouble before it finds them. Good labs and supply rooms post requirements at the door: gloves, eye protection, lab coats—every single time. A safety shower and eyewash station should never be far off. These touches sound basic, but skipping them means gambling with your health. Chemical odor or skin burns don’t wait for a holiday.
After storage, disposal matters just as much. Regulatory agencies take improper dumping seriously—3-nitrophenol can hurt aquatic life if let loose down a drain. I stick with specially marked hazardous waste bottles, and always alert the right waste pickup service instead of tossing leftovers in ordinary trash. Trained staff can neutralize or destroy it safely, keeping people and waterways out of danger.
Whether running a home lab or managing a big facility, protecting people and the environment starts with the habit of careful storage. 3-nitrophenol isn’t forgiving. Investing a few more minutes to double-check containers, room setup, and waste plans protects everyone—and sends a message that we do our work the right way.
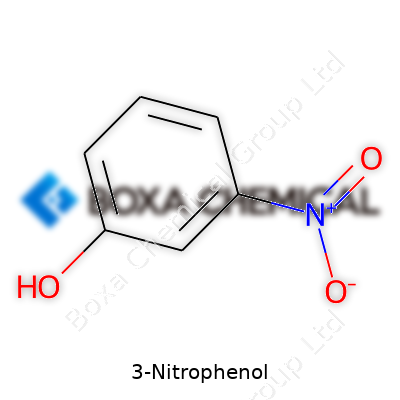
3-Nitrophenol grabs attention because of its direct, no-nonsense structure. Picture a standard benzene ring—six carbon atoms with alternating double bonds, the classic hexagon chemists love to sketch. On this sturdy skeleton, a hydroxyl group (–OH) attaches at the first carbon. Swing three carbons away, you'll spot a nitro group (–NO2) clinging to the third carbon. That’s where the magic of this molecule happens: C6H5NO3.
Chemists look at 3-nitrophenol and see more than just rings and bonds—they see potential. That nitro group changes everything, cranking up the molecule’s acidity and making it more reactive than plain phenol. Acidity in chemistry isn’t just an abstract number. In water treatment and industrial settings, the increased acidity means harsher handling protocols. I’ve seen crew members in chemical plants learn quickly that substances like this don’t play nice if you get sloppy.
The structure tells a story in environmental science, too. That nitro group isn’t content to fade away quietly. 3-Nitrophenol can stick around in soils and waterways thanks to its resistance to breakdown. From firsthand experience working with water testing teams, I know it’s not rare to find traces near old dye factories or in urban runoff. Its stability forces waste management teams to rethink their disposal and filtration methods, pushing for stronger containment and treatment reactors.
Now consider the industries where 3-nitrophenol shows up. In the past, dye manufacturers depended on its sharp chemical edge to make colorants last longer. Agrochemical companies saw promise in the molecule’s structure for making pesticides more potent. Stability may sound good for shelf life, but it never feels great when that same stability means toxic residues linger in the environment. The Environmental Protection Agency categorizes compounds like 3-nitrophenol as pollutants of concern, and for good reason. Researchers warn that persistence brings dangers for aquatic life, especially since the molecule disrupts some biological pathways in fish and bacteria.
Ignoring safety concerns is never an option. In places I've visited, good chemical practice starts with respect for the molecule’s acidity and toxicity. Workers rely on strong gloves, goggles, exhaust hoods, and careful storage. Regular monitoring of water and soil, not just at chemical plants but along rivers downstream from big cities, makes a clear difference. Beyond reaction and remediation, the push now is for greener chemistry. Labs experiment with alternatives that break down faster or swap out the nitro group for options that don’t stick around in the environment.
For those learning about 3-nitrophenol in classrooms or labs, remember that each part of its structure shapes how it behaves out in the world. Every nitro group or hydroxyl side chain carries responsibilities. Real progress rests in understanding both the raw chemistry and the way choices ripple into communities and ecosystems. Whether in a beaker or beneath a microscope, paying attention to those details leads to safer practices and smarter innovations in the future.
3-Nitrophenol appears as a light-yellow crystalline solid that’s often recognized by its sharp, somewhat medicinal smell. It tends to melt at around 97 degrees Celsius and stays solid under normal room temperatures, which matters for storage and handling. Water can dissolve it, but not completely—there’s only so much that will mix before undissolved crystals start collecting at the bottom of a glass. Outside of water, ethanol and ether do a much better job pulling it into solution, something any chemist working in a lab soon discovers.
This compound stands apart thanks to the nitro group sitting on the third carbon of the benzene ring. That little detail, which seems small on paper, throws off the balance of the molecule in ways you really notice in practical chemistry. The presence of both a hydroxyl group and a nitro group changes how it looks and reacts compared to other phenols. This structure means 3-Nitrophenol shines bright yellow under UV light and absorbs ultraviolet light strongly—useful details for anyone trying to identify it or track its presence in a sample.
3-Nitrophenol doesn’t just sit there on the shelf; it jumps into reactions readily. This compound is more acidic than most phenols because the nitro group yanks electronic density out of the ring. That makes it easier to lose a hydrogen ion—a fact that shapes how it interacts with bases and even water, compared to old-school phenol. In practical terms, if you drop 3-Nitrophenol into an alkaline solution, you’ll see a shift in color as it changes forms.
Manufacturers and researchers use its reactivity, but that same quickness brings challenges. If you ever spill it, it’s not something you brush aside. Inhalation or skin contact can lead to irritation, breathing trouble, or worse after prolonged exposure. Safety goggles, gloves, and decent ventilation aren’t just guidelines—they’re non-negotiable. People tend to downplay risks, but plenty of accident reports start with someone in a hurry.
Long-term, environmental release deserves a closer look. 3-Nitrophenol breaks down in soil and water with some help from sunlight and microbes, but not fast enough for comfort in industrial spill scenarios. It can pose risks to aquatic life before it finally degrades, and water testing teams often look for it as part of pollution screens near factories.
The punch packed by 3-Nitrophenol’s chemical structure means it shows up in more places than you might imagine. Dye makers and pharmaceutical companies turn to it as an intermediate—basically, a stepping stone when building more complicated compounds. Pesticide chemists rely on nitration patterns for selectivity, and this molecule fits the bill.
Lab routines usually take this stuff seriously. It burns the hands and nose. In one research stint, handling buckets of phenols hammered that lesson home—nitrated ones demanded an even higher level of caution. Chemists knew the cost of sloppiness didn’t just mean ruined experiments but also putting health at risk.
Control starts at the source. Facilities storing or using 3-Nitrophenol benefit from tightly sealed containers, air filtration, and clear spill protocols. Eliminating leaks and unused leftovers lowers risks for people and local ecosystems. Training goes a long way; seasoned colleagues often share tips for keeping exposure down and reactions safe. For the waste that does leave the building, chemical treatment and professional disposal remain standard—never down the drain or in the trash.
Real progress comes through responsible sourcing and regular monitoring. Factories and labs need ethical supply chains and tight discharge practices. Regular assessment of nearby water and soil helps catch contamination before it grows into a crisis. Advances in alternative chemistries might someday phase out nitrophenols for certain applications, but in the meantime, practical respect for their properties keeps both people and the environment safer.
| Names | |
| Preferred IUPAC name | 3-nitrophenol |
| Other names |
m-Nitrophenol meta-Nitrophenol 3-Hydroxynitrobenzene |
| Pronunciation | /ˌθriːˈnaɪtrəʊˌfiːnɒl/ |
| Identifiers | |
| CAS Number | 100-00-5 |
| Beilstein Reference | 1209227 |
| ChEBI | CHEBI:22139 |
| ChEMBL | CHEMBL15608 |
| ChemSpider | 68438 |
| DrugBank | DB06757 |
| ECHA InfoCard | 100.009.038 |
| EC Number | 1.1.1.64 |
| Gmelin Reference | 82253 |
| KEGG | C01580 |
| MeSH | D014931 |
| PubChem CID | 980 |
| RTECS number | SN1225000 |
| UNII | D8R6Z8C77G |
| UN number | UN2026 |
| CompTox Dashboard (EPA) | EPA CompTox Dashboard (3-Nitrophenol): DTXSID2022597 |
| Properties | |
| Chemical formula | C6H5NO3 |
| Molar mass | 139.11 g/mol |
| Appearance | Yellow crystalline solid |
| Odor | Odorless |
| Density | 1.479 g/cm3 |
| Solubility in water | 17 g/L (20 °C) |
| log P | 1.91 |
| Vapor pressure | 0.00016 mmHg at 25°C |
| Acidity (pKa) | 8.36 |
| Basicity (pKb) | 9.38 |
| Magnetic susceptibility (χ) | -77.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.613 |
| Viscosity | 1.315 cP (25°C) |
| Dipole moment | 3.60 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 121.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -40.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1596 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D08AX06 |
| Hazards | |
| GHS labelling | GHS02, GHS06 |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H332, H335 |
| Precautionary statements | P260, P264, P270, P273, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0-OX |
| Flash point | 130 °C |
| Autoignition temperature | 540°C |
| Explosive limits | Lower: 2.3% Upper: 10.2% |
| Lethal dose or concentration | LD50 (oral, rat): 282 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 282 mg/kg |
| NIOSH | SN4550000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of 3-Nitrophenol is: "5 mg/m³ (as total dust) |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
3-Nitroaniline 3-Nitrobenzaldehyde 3-Nitrobenzoic acid 3-Nitrochlorobenzene 3-Nitroanisole |