Scientists first stumbled onto catechol derivatives during efforts to break down plant-based substances in the 19th century. With coal tar’s complicated soup of chemicals under the microscope, chemists started cataloguing every phenolic fragment, including methyl-substituted versions. Animal and plant metabolism revealed even more of these offshoots, encouraging research into their natural and artificial synthesis. 3-Methylcatechol’s name crops up throughout old organic chemistry papers, usually in the context of aromatic ring modification, pesticide breakdown, or lignin analysis. Its importance grew as industries began seeking out specialty chemicals for antioxidants, flavor enhancements, and intermediate steps in fine chemical production.
3-Methylcatechol belongs to the catechol family. Its molecule supports two hydroxyl groups clinging to a benzene ring, with the methyl group crowding at the third carbon. Chemical factories turn out this white-to-light brown solid for everything from pharmaceutical intermediates to research reagents. You find it under many trade names, shipped in sealed drums, or as a lab-grade crystalline powder. Its price swings reflect how tricky synthesis and high-purity requirements can be. The world market doesn’t treat it as a commodity, but it keeps a steady role in sectors where precision and reactivity matter.
3-Methylcatechol usually shows up as slightly off-white crystals with a sharp, phenolic odor. It melts near 108°C, boils around 264°C, and dissolves well in alcohols and water. Its molecular weight sits at 124.13 g/mol. It brings classic catechol chemistry to the table: both hydroxyl groups activate the ring, boost solubility, and make the compound sensitive to air, light, and oxidation. Handling it often leaves the user tracing a faint medicinal scent, and a half-used bottle quickly turns dark from exposure to oxygen. This kind of sensitivity calls for dark, airtight storage and quick work in the lab.
Most chemical suppliers stamp 3-methylcatechol containers with batch analysis details: purity (often above 98%), melting point, moisture content, and trace metal analysis. Certificate of Analysis paperwork trails each canister, making lab audits smoother. Storage advice warns against heat, direct sunlight, and open air due to the compound’s ability to oxidize. Labeling must comply with local chemical safety standards, including United Nations transport codes, hazard pictograms, and chemical abbreviation (3-MC or 3-MCATECHOL). Pharmacopeia listings, where relevant, detail minimum and maximum allowable impurities and solubility specifications for clinical or research applications.
Laboratories synthesize 3-methylcatechol in several ways, though most involve methylating catechol directly or demethylating 3-methylanisole through catalytic hydrogenation or hydrolysis. Friedel–Crafts alkylation of catechol provides short routes on a small scale, but overalkylation and tar formation need careful attention. Some older patents recommend oxidative demethylation using strong acids and mild oxidants to peel the methyl group from its precursor. Yield and purity swing with operator skill, making it necessary to start with high-purity reagents and monitor every step. Scaling production to industrial volumes always brings headaches—especially the challenge of controlling side reactions and separating close-boiling impurities.
Once synthesized, 3-methylcatechol serves as a flexible platform for modifications. Its two adjoining hydroxyls invite esterification, etherification, and oxidation, turning out a variety of derivatives with changing solubility or biological activity. Coupling reactions introduce heavier groups, producing antioxidants or pharmaceutical starting points. Nitration and halogenation give new flavors to the aromatic core, but usually require careful temperature control to avoid degradation. Oxidative coupling or polymerization transforms it into brownish humic-type substances, which bear a resemblance to lignin breakdown products found in soil and natural waters. Chemists have used these reactions to build libraries of related compounds for screening in the drug discovery process.
Chemical catalogs often list 3-methylcatechol as 3-methyl-1,2-benzenediol, m-cresol catechol, or 3-methylpyrocatechol. Older literature sometimes shortens it to 3-MC. Trade chemicals coming from Japan, the US, and Europe may feature brand names based on their respective suppliers. Whatever the label, the structure stays the same—marked by the essential catechol diol skeleton with a lone methyl branch.
3-Methylcatechol doesn’t fall under "everyday use" chemicals. Even trace exposure can trigger skin irritation, breathing problems, or headaches. Shutdowns in plant settings almost always occur if sensors pick up airborne phenols. Standard protocols require gloves, goggles, lab coats, and sometimes respirators. Spill kits include neutralizers and absorbent pads, and waste goes straight to hazardous material disposal. Chronic exposure studies point to potential liver and kidney stress in animals, and regulatory agencies screen occupational settings frequently. Anyone handling it spends time drilling emergency procedures and reviewing Safety Data Sheets (SDS) before opening the bottle.
Research labs prize 3-methylcatechol for studying enzyme reactions and antioxidant mechanisms. Pharmaceutical developers use it in multi-step syntheses as an intermediate. Environmental chemists monitor it in water samples when tracking the breakdown of herbicides or aromatic hydrocarbons. Certain flavor chemists test its trace derivatives in food analysis, scanning for the signals of spoilage or fermentation. Some industrial sectors, especially makers of fine chemicals or specialty polymers, test blends using methylcatechol derivatives to improve performance under oxidative stress. Its niche role in these spaces comes from the combination of reactivity and sensitivity, rather than sheer production volume.
Lab notebooks fill up with projects aiming to harness the reactivity and structure of 3-methylcatechol. One wave of research circles around oxidative enzymes, using it as a substrate in laccase, peroxidase, and tyrosinase assays. Structure-activity studies link its methyl group positioning with anti-inflammatory or antimicrobial activity. A string of patent filings in recent years shows chemical startups looking at advanced antioxidants made from methylcatechol cores for lubricants and plastics. Academic collaborations probe its breakdown in soils under sunlight, feeding climate and pollution models. Each research group brings a new twist, tracking how even small changes in structure unlock new pathways for chemistry and biology alike.
Lab animal studies chart out the toxicological landscape. Researchers link acute exposure to nervous system symptoms in rodents, with high doses producing tremors and weight loss. Chronic exposure links back to mild liver and kidney changes, often reversible after stopping treatment. Skin studies flag it as an irritant, and concentrated vapors may trigger coughing and headaches. Environmental work highlights its persistence in water and tendency to react with sunlight and oxygen, breaking into less studied byproducts. Regulatory discussions focus on workplace exposure, drinking water safety, and factory emissions. Anyone looking for a deeper safety margin weighs the pros and cons of using methylcatechol versus safer or more stable phenolic compounds.
3-Methylcatechol’s future connects with ongoing innovation in green chemistry, pharmaceuticals, and environmental testing. As demand for safer antioxidants and stronger stress-resisting materials rises, chemical firms revisit old molecules like methylcatechol, hoping that small improvements in synthesis and purity will unlock profitable new routes. Environmental chemists continue adapting analytical techniques, hoping to fingerprint its environmental fate more accurately in river and soil samples as regulatory agencies tighten control on phenolic byproducts. In academic labs, new enzymatic methods and biotransformation tricks hint at greener, less wasteful routes for producing and modifying methylcatechols. Every improvement promises to widen the field of applications—from better drugs to more effective chemical sensors and smart materials engineered to withstand oxidative abuse.
Step into any research lab with a focus on biochemistry or environmental toxicology, and you’ll notice shelves lined with odd-sounding chemicals. 3-Methylcatechol stands out among them, not just for its mouthful of a name. Scientists rely on it to mimic substances found in the environment—helping them break down what happens to pollutants, drugs, and even plant compounds as they journey through our ecosystem or our bodies.
I remember my own days hunched over lab benches, watching enzymes convert molecules right under the microscope. 3-Methylcatechol often ended up in our beakers. Chemists value it as a model compound when studying how enzymes called catechol dioxygenases chew up aromatic rings. These enzymes don’t just float in labs; bacteria use them in soil and water every day, cleaning up oil spills or pesticide runoffs one molecule at a time. By tracking 3-methylcatechol’s breakdown, researchers uncover how nature might deal with synthetic chemicals or leachates in real scenarios.
Pollution isn’t an abstract problem for most communities. Rivers absorb agricultural chemicals, cities deal with groundwater contamination, and toxic byproducts drift through the air. 3-Methylcatechol stands in as a chemical cousin of phenols and catechols found in real-world pollution. By experimenting with it, environmental scientists watch how bacteria and fungi degrade toxic messes—information that shapes clean-up efforts everywhere from farm fields to Superfund sites. The compound isn’t just a stand-in for pollution research; its activity signals how nature might slow or speed chemical decay, affecting soil health, water purity, and public safety.
Medicinal chemists and toxicologists depend on 3-methylcatechol for a different reason. Drug development relies on predicting how the body—and the environment once drugs break down—deals with each new molecule. 3-Methylcatechol sneaks into experiments as a byproduct or as a key building block. Its interactions with enzymes like cytochrome P450 help forecast how a new medication will break apart once swallowed. Some cancer treatments and anti-inflammatory drugs would stall in development without this basic chemistry. It’s one thing to create a drug that works in a petri dish; it’s another to know what happens to every piece after the body is done with it. This insight protects people from toxic side effects or environmental harm after excretion.
3-Methylcatechol’s benefits can bring risks, too. Handle it carelessly, and you run into the same toxicity issues that make catechols dangerous in industrial spills—irritation, possible long-term health impact. This isn’t a warehouse chemical for casual use. Labs and manufacturers stick to strict transport and disposal rules for a reason. Transparency around these practices builds trust, not just for workers, but for the neighborhoods and water systems that could face fallout from bad management.
Safer alternatives and improved handling protocols would go a long way. Supporting fundamental research means innovations in green chemistry soon spill over into industrial process improvements, reducing the environmental toll even further. In short, practical chemistry around 3-methylcatechol shapes not only cleaner water and safer drugs, but a more thoughtful way of living with the chemicals developed in the name of progress.
Anyone who works around chemicals knows that a little caution goes a long way. 3-Methylcatechol, often found in research and chemical manufacturing, carries its own risks that can’t be brushed aside. Having spent some time in labs with volatile organics, I’ve seen firsthand how accidents happen less with rules, and more with habits. People who pay attention, ask questions, and learn from one another end up staying safe. So let’s talk about what matters most with this compound.
3-Methylcatechol may look unremarkable—usually a pale solid, faintly aromatic—but its impact builds quietly. Skin irritation and allergic reactions are common if you get careless. I’ve watched someone wipe sweat from their brow without gloves, then regret it almost instantly. Eyes aren’t any better suited to this chemical, since even a small splash causes stinging and redness.
Inhaling fine dust or fumes risks coughing, shortness of breath, or even long-term issues. Chronic exposure, though less flashy, stays on a chemist’s worry list for a reason. It takes discipline to remember that just because you can’t see damage doesn’t mean it isn’t happening.
Personal experience and what occupational health experts say line up: never shortcut personal protective equipment (PPE). Well-fitted goggles, chemical-resistant gloves, and a sturdy lab coat make a real difference. You might think a simple surgical mask is enough, but 3-Methylcatechol calls for a fume hood and at least a half-face respirator when powders or vapors are around. I’ve seen cases where even a few minutes of unprotected handling led to days of discomfort.
Clutter feeds carelessness. Clear benches, labeled containers, and easy-access spill kits put everyone at ease. If you can see what you’re doing, and the tools you need are right there, mistakes shrink. Good lighting and space for movement aren’t luxuries—they’re investments in well-being.
Some chemicals just sit in cupboards, but storing 3-Methylcatechol deserves close attention. A dry, cool, well-ventilated spot—away from oxidizers and strong acids—keeps risks down. Leaky lids or loose containers invite bigger problems. I always stick to tightly sealed glass bottles, since plastics sometimes react and turn brittle faster than you’d expect.
Waste means more than pouring leftovers down the sink. Catechols, including 3-Methylcatechol, need specialized disposal. Coordinating with environmental health or hazardous waste crews makes sense, since pouring out the wrong way pollutes water and puts sewage workers at risk. Labels shouldn’t fade or peel—permanent markers, tough waterproof stickers, and clear chemical names help everyone stay honest and sharp.
Even thoughtful people slip up. Eyewash stations, emergency showers, and fresh air access must be more than theory. Running regular drills builds confidence. My mentor once said, “You’ll only reach for a fire blanket if you’ve done it before.” It stuck with me, and I’ve noticed younger staff take emergencies seriously when hands-on practice is the norm.
No set of rules alone keeps everyone protected. Open discussion, honest questions, and willingness to share near-misses give a safety culture its backbone. Newcomers—students, interns, or new hires—who feel safe admitting minor mistakes end up learning faster and helping catch bigger problems later. A good team circles back, reviews what went wrong, and adjusts on the fly. In the end, the best handling of 3-Methylcatechol comes down to steady habits, regular dialogue, and the practical commitment to watch each other’s backs.
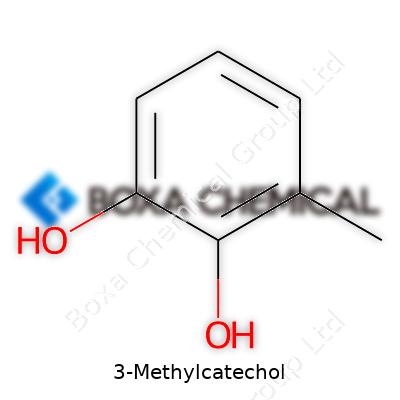
3-Methylcatechol belongs to a family of phenolic compounds known for their presence in the breakdown pathways of lignin, a key component of plant cell walls. Drawing from my own academic exposure in the chemistry lab, I found that small tweaks in molecular structure, such as the placement of a methyl group, can drastically impact the function and reactivity of a compound. 3-Methylcatechol is a clear case. Its formula, C7H8O2, reveals a benzene ring with two hydroxyl (–OH) groups and a methyl (–CH3) group as its hallmark features.
On paper, the structure of 3-Methylcatechol appears simple: a benzene ring with hydroxyls at positions 1 and 2, and a methyl at the 3-position. Chemists say it as 3-methyl-1,2-dihydroxybenzene. The two hydroxyls sit adjacent, lending unique reactivity, while the methyl group gives the molecule its distinct identity. Looking back at my student years, distinguishing these small shifts in position always made the difference between getting answers right or wrong during organic chemistry quizzes.
Moving out of textbooks, 3-Methylcatechol has real significance. It shows up as an intermediate during the microbial breakdown of aromatic pollutants. Certain soil bacteria use it while digesting wood-related waste or even synthetic chemicals released by industry. This point highlights the interconnectedness between basic chemical structure and gigantic environmental cycles. According to work published in Applied and Environmental Microbiology, enzymes from bacteria like Pseudomonas putida act on lignin-based waste, converting it into substances like 3-Methylcatechol as part of nature’s recycling system.
There’s growing attention on how chemicals like 3-Methylcatechol might impact people and nature. In the lab, its reactivity poses safety risks; it can irritate skin and eyes, and inhalation of dust brings health worries. In the wider world, though, it takes on a different role—helping scientists understand how to harness microbes for breaking down pollution. The key here lies in learning from these natural systems rather than fearing the chemistry. Government agencies and universities have begun investing in research to understand not just the risks but also the ways beneficial microbes turn waste into useful byproducts.
Keeping people safe takes clear rules in the lab and in industry. After working in a university research group, I came to respect the simple routine of using gloves, goggles, and a fume hood. These habits mean fewer accidents—no matter how harmless a chemical seems. In industry, proper disposal of waste containing chemicals like 3-Methylcatechol stands as a basic requirement. Environmental agencies recommend regular monitoring and adoption of biodegradation strategies, relying on bacteria to neutralize hazards before they reach waterways.
Looking at 3-Methylcatechol, its formula and structure reveal more than a string of atoms; they point toward stories of biodegradation, health safety, and environmental responsibility. By paying closer attention to details in the lab and drawing from current research, society can turn challenges in chemistry into opportunities. Exploring how microbes transform 3-Methylcatechol in soil fuels both curiosity and the hope of finding cleaner ways to deal with chemical waste.
3-Methylcatechol pops up in labs as both a research chemical and a building block for other compounds. Its powder form makes it seem just like another chemical jar on the shelf, but it packs more risk than most would expect. The substance can irritate the skin, eyes, and respiratory tract, plus it poses an environmental risk. Ignore proper storage and small mistakes can escalate quickly. I’ve seen simple carelessness, like leaving a cap off or allowing containers to drift near heat sources, turn into bigger emergencies. Sometimes, it’s the overlooked materials that create the whole mess in the first place.
The goal is to keep 3-Methylcatechol stable and safe to handle, start to finish. You want it locked tight in a cool, dry, and dark spot. Even sunlight’s warmth or a nearby heating vent can trigger chemical changes nobody asked for. Chemical storerooms with climate control do the job, but I’ve managed well in smaller spaces by just keeping containers in a secure, designated cabinet. Forgetting that simple step gets people in trouble more often than you'd think.
Glass is the container material of choice—plastic sometimes reacts or lets invisible vapors seep out over time. Lids must fit securely. For extra peace of mind, using secondary containment tubs can catch leaks or spills early before they reach something combustible or sensitive. My first year working with chemicals, I learned quickly that nobody wants to mop up a sticky spill at the bottom of a shelving unit.
Clear, accurate labels matter. I always mark not just the name but concentration, date received, and the assigned hazard class. Labs that update their inventory logs regularly avoid surprises. It might sound basic, but knowing exactly what’s on hand fixes more problems in advance than elaborate safety protocols. Tracking helps you rotate stock, spot deteriorating containers, and plan disposal before a shelf become hazardous waste all by itself.
Good ventilation isn’t only for when you’re pouring or weighing out chemicals. The storage area benefits, too. Using chemical-resistant shelving and separating organics, oxidizers, and corrosives keeps incompatible chemicals from mingling. I’ve seen incidents where two completely separate projects were derailed by a misstep at the storage shelf. Newcomers often underestimate how vapors or accidental spills drift between containers if space gets tight.
No labeling system or fancy storage cabinet beats a crew that knows what to do if things go sideways. I encourage every staff member to review safety data and rehearse spill or exposure responses. Having clean-up kits, eyewash stations, and clear signage in place means people respond faster. You might never need the training, but if something spills or a container cracks, you can bet you’ll wish everyone knew the routine by heart.
Disposing of leftover 3-Methylcatechol isn’t as simple as tossing it in the trash. Regulations ask for chemical waste experts to collect and process it. Mixing it with household waste risks community safety and pollutes groundwater. Experienced handlers plan disposal pickups well in advance of expiration dates. That small act keeps the workplace and the world outside safer for everyone.
Real safety lives in details: check lids, use the right containers, keep chemicals separated, and never improvise with risky substitutes. I’ve learned that shortcuts with chemical storage almost always lead to bigger issues down the line. Taking the extra minute for careful storage means smoother workdays, greater confidence in safety, and fewer headaches tomorrow.
3-Methylcatechol pops up in research labs and some industrial processes. I’ve seen its label pop up during lab safety briefings, always highlighted in bold. This isn’t a compound you find sprinkled around kitchen cupboards—handling it calls for gloves, goggles, and good ventilation. People often group it with other catechols, but it carries its own set of risks, enough to raise a couple of red flags for anyone who deals with chemicals.
My own time in a university chemical storeroom made me wary of bottles marked with hazard symbols. On exposure, 3-Methylcatechol can irritate eyes, skin, and the respiratory tract. Animal studies flag up possible adverse effects after short-term and long-term exposure, suggesting potential harm to organs after repeat doses. Studies on catechol derivatives have shown possible links to changes in the liver and kidneys. Handling dusts and vapors seems particularly risky. Workplaces prioritizing safety always list this compound as harmful if inhaled or absorbed through the skin.
Acute toxicity numbers speak for themselves. It's not the deadliest chemical in the world, but the margin for error narrows for lab techs, warehouse staff, and anyone washing out glassware. Accidents and spills without quick intervention often lead to headaches, dizziness, and other discomforts. Strong evidence suggests it can form reactive intermediates under certain conditions, potentially leading to cell damage.
Concerns about water contamination often surface during environmental briefings. Its structure allows it to linger in aquatic systems and can affect fish and aquatic organisms at low concentrations. Studies point out that catechols, including 3-Methylcatechol, disrupt normal biological functions in water fleas and other small animals. Anyone familiar with chemical waste protocols knows how important it is to collect and treat wastewater, not just dilute and dump down the drain. Wild stories from wastewater workers remind me of just how easily overlooked compounds slip into rivers.
Most people avoid direct contact by following standard lab and industrial practices: tightly sealed containers, proper labeling, and segregation from oxidizing agents. Spills and leaks always seem to happen at the worst possible moments, so absorbent materials and neutralizers sit handy. Companies facing audits often invest significant effort in secondary containment and emergency training. My habit of re-checking container seals before leaving for the day comes from personal experience—nothing ruins a week like discovering a slow leak on a high shelf.
Reducing risk relies on clear information for workers and a culture of reporting near-misses. Investing in local exhaust ventilation, emergency showers, and eye wash stations minimizes the impact of mistakes. Regular review of safety data sheets keeps crews updated on best practices. Companies and universities can take small steps, like hosting monthly safety talks and keeping chemicals in restricted areas, and those steps pay off with fewer incidents.
Calls for tighter regulation often come from communities near manufacturing plants. Complying with local and international chemical safety laws—such as OSHA’s Hazard Communication Standard—helps protect workers and neighborhoods. When facilities share risks and controls, public trust climbs, and through that openness, both safety and environmental protection gain strength.
| Names | |
| Preferred IUPAC name | 3-methylbenzene-1,2-diol |
| Other names |
3-Methylcatechol 3-Methyl-1,2-benzenediol 3-Methylpyrocatechol Pyrocatechol, 3-methyl- 1,2-Dihydroxy-3-methylbenzene |
| Pronunciation | /ˌθriːˌmɛθɪlˈkætɪˌkɒl/ |
| Identifiers | |
| CAS Number | [Catechol] 3-Methylcatechol CAS Number: **[4881-44-7]** |
| Beilstein Reference | 1209237 |
| ChEBI | CHEBI:34219 |
| ChEMBL | CHEMBL18156 |
| ChemSpider | 59060 |
| DrugBank | DB04051 |
| ECHA InfoCard | 100.041.196 |
| EC Number | 205-028-7 |
| Gmelin Reference | 82848 |
| KEGG | C06555 |
| MeSH | D019294 |
| PubChem CID | 6945 |
| RTECS number | GG9600000 |
| UNII | 7G1Y1K44TI |
| UN number | UN2759 |
| Properties | |
| Chemical formula | C7H8O2 |
| Molar mass | 124.14 g/mol |
| Appearance | White to light brown solid |
| Odor | phenolic |
| Density | 1.102 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.88 |
| Vapor pressure | 0.0195 mmHg (25°C) |
| Acidity (pKa) | 9.24 |
| Basicity (pKb) | pKb = 10.10 |
| Magnetic susceptibility (χ) | -72.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5430 |
| Viscosity | 1.897 mPa·s (25°C) |
| Dipole moment | 1.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 137.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -123 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3410 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H301, H311, H331, H373 |
| Precautionary statements | Precautionary statements of 3-Methylcatechol: "P210, P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 116 °C |
| Autoignition temperature | 540 °C |
| Explosive limits | Explosive limits: 1.1–7.1% |
| Lethal dose or concentration | LD50 (oral, rat): 391 mg/kg |
| LD50 (median dose) | LD50: 283 mg/kg (rat, oral) |
| NIOSH | SN1575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Methylcatechol: Not established |
| REL (Recommended) | 0.1 mg/m3 |
| IDLH (Immediate danger) | Not established |