Chemists first took an interest in 3-methoxycatechol back in the first half of the 20th century, driven by curiosity over phenolic compounds found in plants. Early studies focused on breaking down lignin, the tough fiber in wood, and out popped this intriguing molecule as a byproduct. Scientists in classic European research centers and later at US labs treated plant extracts with various reagents, keeping careful watch as pure crystals formed. Over decades, folks learned that this compound—also known as 3-methoxy-1,2-benzenediol—had more to offer than just a spot on a chalkboard. Modern research points to both natural pathways, like the metabolism of vanillin in the body, and synthetic approaches, opening new doors in understanding structure and function.
3-Methoxycatechol shows up as an off-white or slightly beige powder. Most people handling it come across it through chemical suppliers, and those little glass vials line the benches of academic labs from Tokyo to Berlin. The compound’s structure carries a benzene ring with two neighboring hydroxyl groups (think: catechol), and a methoxy group tucked at the third position. Companies often list it under different labels, reflecting the global reach of chemical trade and the overlapping claims of nomenclature. Researchers chase this compound for its anti-oxidant action, its potential medical applications, and its unique behavior in organic synthesis. Compared to plainer catechols, that methoxy twist brings out new reactivity and solubility—helpful qualities depending on what problem needs solving.
You can recognize 3-methoxycatechol by its fine crystals, which melt reliably around 151°C. It dissolves well in ethanol and warm water, leaving only a faint tint if anything. Its mild, slightly sweet odor betrays its phenolic roots. Reliability in melting point helps confirm purity—a habit drilled into the heads of every organic chemist when confirming bench work. The molecule resists light oxidation better than its parent catechol, so it rarely browns up in storage if kept dry and tight-lidded. This small stability bump can matter in real-world storage and shelf-life planning, because air and moisture offer two silent enemies to small aromatic molecules.
Suppliers label containers clearly with purity (usually 98% or greater by HPLC/GC), molecular weight (140.15 g/mol), CAS number (621-37-4), batch number, and standard safety data. Handling advice reflects best practices drawn from decades of mishaps: goggles, gloves, and a working fume hood for open transfers. Labels typically describe storage targets: cool, dark, sealed. Tech sheets from leading catalogues spell out every specification, from particle size (not always essential in most uses, but helpful for formulation) to residual solvent limits. Experienced chemists keep one eye on these sheets, since a stray contaminant can turn a promising reaction into a chemist’s headache.
Classic methods involve methylating catechol, sometimes with dimethyl sulfate in a basic medium. Whether prepared under batch or flow conditions, one faces the age-old challenge of selectivity: too harsh a base or excess methylating agent, and multiple mono- and dimethoxy products creep in. Some routes favor milder agents like methyl iodide to tone down those side reactions. For large-scale jobs, eco-friendlier approaches use green solvents like water or ethanol, though yields can be a struggle. Extraction and column purification polish things up for research or pharmaceutical grade, but anyone scaling up has to balance purity, yield, and safety. Advances from the last fifteen years showcase microwave reactors, ionic liquids, and even enzyme-catalyzed pathways, each chipping away at energy and waste without sacrificing the result.
Chemists tinker with this molecule often, drawn to its orthogonal reactivity. The double hydroxyls open doors to coupling reactions, such as Suzuki or Ullmann, sidestepping the methoxy group’s shield. Nitration proceeds smoothly, but only after protecting those reactive hydroxyls. In medical labs, 3-methoxycatechol works as a starting point for anti-cancer and anti-microbial analogs, especially when the methoxy group morphs into bulkier ethers, or is replaced with hydrophilic chains for drug delivery. Folks in material science see promise too, since the aromatic core can slip into polymers, bringing extra function like adhesion or UV resistance. Everyone loves a molecule that doesn’t just sit in a bottle, but steps up as a flexible building block.
Go through catalogs in Europe, North America, or Asia, and you might see 3-methoxycatechol listed as 1,2-dihydroxy-3-methoxybenzene, 3-methoxy-1,2-benzenediol, or even m-guaiacol. The world of chemical synonyms often confuses non-specialists, which sometimes leads to delays and procurement errors. Each supplier adds its own flair, mixing IUPAC with traditional shorthand. Anyone ordering needs a careful eye—those swap-outs between ‘methoxy’ and ‘guaiacyl’ groups change more than spelling, they risk misidentification. Cross-referencing CAS numbers (621-37-4) is the surest path in a sea of nearly-named twins.
Handling aromatic polyphenols like 3-methoxycatechol demands respect. Contact with skin can lead to irritation, especially after repeated or prolonged use. Inhalation risks increase during powder handling or open synthesis, so everyone ought to treat it as a mild irritant with room for worse if neglected. Labs post clear signage and maintain spill kits to catch the rare accident: absorbent pads, gloves, and neutralizer handy. Fire doesn’t often start from pure storage, but exposure to strong oxidizers raises risk—a lesson hammered home in more than a few fire marshal reports. Safety data sheets point out environmental hazards too, flagging suspected aquatic toxicity, so draining waste into the sink means breaking both rules and habits. Good practice comes down to diligence, a decent fume hood, and that nagging sense to double-check seals and containers before locking up.
Pharmaceutical companies explore 3-methoxycatechol for its antioxidant and anti-inflammatory abilities. In preclinical trials, lab animals treated with derivatives showed real promise against oxidative stress and certain tumors. Beyond the research bench, environmental labs look at this compound as a marker for lignin breakdown, yielding clues about wood processing and even forest fires. Synthetic chemists covet the molecule for custom polymer design, hoping to craft better adhesives, coatings, or sensors with a tweak in the aromatic ring. Dermatologists and cosmetic chemists pull it into discussions about melanin formation and pigment regulation—in some places, as a regulator of tyrosinase. Biological researchers see this as more than a tool; they use it to probe enzyme pathways, seeking the secrets behind plant resilience and aging.
The last decade saw a surge in structural modifications of 3-methoxycatechol. Collaborative research groups work on targeted delivery, attaching linkers or nanoparticles to tread new ground in drug development. Medicinal chemists chase broad trends: anti-cancer, anti-viral, and neuroprotective projects, matching in vitro findings with translational hopes. Analytical chemists refine detection methods, developing ultra-sensitive mass spec routines to follow metabolism. Investment in green synthesis unlocked milder, less polluting routes, always valuable to those scaling up. Each R&D breakthrough triggers a cascade of patents, many housed in biotech incubators or start-up portfolios. Teams test combinations of 3-methoxycatechol with classic drugs, searching for synergy or reduced toxicity. In industry, every new derivative brings both promise and fresh regulatory hurdles, so keeping up with the literature and pipeline updates means more than just curiosity—it means staying competitive.
Toxicologists keep a wary eye on 3-methoxycatechol’s effects. Animal models exposed to high doses show changes in liver enzymes and renal markers, prompting limits on research or potential therapeutic use. Slight irritation on contact remains the most common issue, but larger questions about mutagenicity linger. Early cell-based studies flag concern for DNA damage, especially after prolonged exposure and in metabolically active tissues. Inhalation studies highlight caution for workers in manufacturing or large-scale synthesis. Any clinical application faces tight scrutiny from regulatory agencies; risk assessment routines feed off chronic exposure data, always balancing possible medical gains with the need for caution. Water solubility raises the stakes for disposal and environmental impact, as aquatic toxicity in fish and microbe assays draws warning notes in safety evaluations.
Moving ahead, scientists expect 3-methoxycatechol research to focus on precise modification for health and materials science. Interest in anti-cancer and neuroprotective applications draws attention and funding, connecting molecular tweaks to disease models and early clinical trials. Surface chemists want to exploit its adhesive properties, hoping to make smarter, more responsive coatings and films. Growing pressure for sustainability in the chemical industry sparks a drive for greener synthesis and wider use of renewable feedstocks, including enzymatic and biocatalytic methods. More researchers now tap into machine learning, using computational predictions to find new derivatives with optimized properties, slashing years off traditional trial-and-error. The molecule itself endures as scientists push the limits of how structure meets function, always aware that success in the lab hinges on discipline, sharp thinking, and attention to the broader impacts.
Every so often, a molecule comes up in conversation among people working in labs or hospitals—a compound that stands out not just for its formula, but for the questions it helps answer. 3-Methoxycatechol is one of those molecules. Under a microscope, it doesn’t look like much. In truth, it packs quite a punch where it counts: medical research and pharmacology. As someone who’s spent time in both academic labs and around clinical research teams, I’ve seen just how valuable it can be.
The search for new medicines often starts with understanding how the body handles stress and inflammation. Many chronic diseases involve oxidative stress—cells attacked by unpleasant molecules called free radicals. Our bodies can fight back, but they don’t always win. Here’s where 3-Methoxycatechol finds its groove. It belongs to a family called catechols, which are often studied for their antioxidant properties. In simple terms, this means researchers want to know, “Will this molecule block some of the damage?” The evidence suggests yes, at least in the lab.
Scientists use 3-Methoxycatechol to test its abilities to mop up these harmful radicals. They add it to cell cultures, animal models, or test tubes and measure just how much it slows down or stops oxidative reactions. This helps in building a picture of how future drugs could protect nerve cells, blood vessels, or the heart. That’s not just theory. Studies have pointed out its activity in trapping reactive oxygen species, potentially slowing down the aging process on a cellular level.
This compound isn’t just a tool for theorists. In cancer research, scientists take 3-Methoxycatechol and see what it does to cancer cells, particularly when these cells grow out of control because of oxidative stress. Some experiments show promise, with evidence that this molecule can slow the growth of certain malignant cells. Of course, these are early days—animal models or isolated cells aren’t people—but positive trends matter, especially for diseases like cancer, where every potential lead is valuable.
3-Methoxycatechol also shows up as a reference standard when checking other drugs or samples in quality control. Consistent results allow regulators and doctors to trust the medicines and supplements that get shipped out. In labs I’ve worked in, running a batch of tests with a clear standard like this one helps diagnose problems faster, leading to safer results for patients.
One thing that keeps coming up with compounds like 3-Methoxycatechol is the need for responsible use. It may have promise on paper, but jumping straight from lab results to claims about cures won’t cut it. Anyone selling supplements based on preliminary studies invites problems. We need solid, well-conducted clinical trials. That’s what builds trust. The way forward looks clear: closer team-ups between chemists, biologists, and healthcare professionals will drive better solutions—and at the end of the day, hopefully give people more reasons to hope.
Years spent around research benches show that every molecule has a story, some longer than others. 3-Methoxycatechol’s story keeps growing, partly because it helps fight the kind of damage that underlies many big health challenges. Used wisely, it can shape better answers for tomorrow’s patients—if we keep asking the right questions today.
Walking through a lab in the early days of my research career, it was easy to get lost in a sea of chemical names, strange acronyms, and cryptic formulas. Some names pop up more often than others because they hide important stories, sometimes about health, sometimes about nature, and sometimes about new technology. 3-Methoxycatechol happens to be one of those names that matter in the world of chemistry and biology, not because it rolls off the tongue but because its formula, C7H8O3, sits at the crossroads of plant science, medicine, and even environmental studies.
The architecture here isn’t just a string of letters and numbers. C7H8O3 tells us this molecule packs seven carbons, eight hydrogens, and three oxygens — arranged in a particular pattern that chemists recognize as a cousin of catechol, with a methoxy group set apart from the usual duo of hydroxyl groups. It is this configuration that gives 3-Methoxycatechol its signature moves. In everyday research, structure shapes identity far more than a name ever could.
Many plant-based compounds pop up in natural defense, disease resistance, or even as part of a tree’s way of communicating with its neighbors. 3-Methoxycatechol emerges from those botanical pathways, especially from the breakdown of lignin, the tough material that gives plants their backbone. Research papers and practical work reveal its presence in fruits, vegetables, and sometimes in smoke from burning wood, all of which hints at its reach well beyond any single experiment. Its antioxidant power plays into the longstanding hunt for natural molecules that might shield human cells from damage linked to aging or pollution-induced stress. In the lab, this molecule gets tested for antibacterial and anticancer effects, not because there’s hype, but because evidence often shows real, measurable activity against problem microbes and some cancer cell lines.
Experience tells us that once you reach molecules like 3-Methoxycatechol, something else happens. You begin to see the links between plant biology and drug discovery. Simple white powders on a bench lead to new ideas for drugs that could slow disease or help resolve infections—reminding me of many long afternoons running assays and tracking color changes on microplates. Modern science leans on such small, distinct molecules to light the path towards therapies that don’t just chase symptoms but go after root causes.
The fact that C7H8O3 comes from both nature and the lab shines a light on a bigger conversation. Natural products often sit at the start of drug discovery journeys, partly because they have already survived natural selection. Plants, after all, can't run away, so they invent chemistry to handle threats. By studying and copying these molecules, researchers keep the process safer and more sustainable. There’s heavy pressure to avoid synthetic chemicals that weigh down ecosystems or introduce toxic residues into water and soil. Focusing on molecules like 3-Methoxycatechol represents a move toward green chemistry. Sourcing, synthesizing, and applying these compounds thoughtfully limits collateral damage and keeps progress tangible, not just theoretical.
The formula of 3-Methoxycatechol, C7H8O3, may seem like the realm of specialists, but its story stretches into agriculture, pharmacology, and environmental work alike. Seeing this firsthand changes any cold view of chemistry into something deeply connected with daily health, food, and the ongoing search for safer technologies.
Walk through any chemistry lab and you’ll bump into a shelf lined with bottles sporting long, tongue-twisting names. 3-Methoxycatechol doesn’t usually take the spotlight outside scientific circles, but—like so many chemicals—knowing its risks could make a real difference in how researchers and manufacturers handle it.
Studies shed light on how 3-Methoxycatechol interacts with living systems. Through animal testing and cell studies, researchers have spotted signs of toxicity, especially at higher concentrations. This compound can cause irritation to skin, eyes, and airways. Animal studies point toward some negative effects on cell growth and suggest possible links to oxidative stress. Toxic effects aren’t unique here—plenty of phenolic compounds create problems for cells. If 3-Methoxycatechol enters the body in substantial amounts (a rare scenario outside labs or manufacturing), it has a decent chance of causing headaches, dizziness, or more severe impacts.
I remember working in the university lab, where a colleague spilled a small amount without gloves. Redness set in quickly—no mystery about its irritating nature. A 2019 toxicology review identified a moderate toxicity rating, flagging that accidental, routine contact could creep up on people over time.
Some compounds break down quietly in the environment, but 3-Methoxycatechol lingers long enough to spark concern. Its chemical relatives have shown up in studies about aquatic toxicity. Fish and plant life aren’t immune; elevated concentrations can stunt growth or disrupt reproduction cycles. There’s some evidence that exposure in the workplace, particularly in settings where good ventilation is lacking, could raise chronic risks for people working with antioxidants and specialty chemicals.
Handling powders and volatile organics for years, I’ve seen simple precautions pay off. Respirators, proper gloves, and working within a fume hood cut exposure risks by a huge margin. It’s easy to let habits slip, but there’s little margin for error. The International Agency for Research on Cancer has not classified 3-Methoxycatechol as a known carcinogen. Data still points to prudence, especially given the gaps in long-term data.
Shifting from risk to safety starts with information. Training workers and students on safe handling practices pays off far more than installing complex safety systems that get ignored. Labeling and storage matter—some of the most severe incidents stem from mixing up bottles or reusing containers.
Facilities ordering 3-Methoxycatechol have a responsibility. Spills get addressed right away, with clear procedures and straightforward reporting. Selection of personal protective gear isn’t left to chance. In my own experience, teams that run routine risk-assessment drills cut down on accidents, and build a stronger safety culture almost without realizing it.
Waste disposal, too, isn’t just a box to check. Partner with hazardous waste specialists and biological treatment if possible. Reducing runoff into drainage systems protects local water sources, which pays dividends everyone can see. Keep a tight inventory—order only what projects actually use, and rotate stock to prevent degradation or accidental mixing.
Toxic compounds like 3-Methoxycatechol challenge labs and manufacturers to treat safety as part of everyday work, not a line in a manual. Safety organizers who communicate risks—and listen to feedback from frontline workers—often find practical, affordable fixes just by observing real routines. Sometimes, the smallest change in habit makes a lasting difference.
Chemicals with phenolic structures such as 3-Methoxycatechol don’t thrive if you shove them on any old shelf. Over years in the lab, I’ve seen people underrate the importance of storage conditions, and the results often bring more headaches than solutions. This compound gets cranky with air and moisture, so it’s not the sort of bottle you want to ignore.
You’ll find 3-Methoxycatechol reacting with oxygen in air, turning brown or black. That’s more than a cosmetic problem. Oxidized product no longer supports good science, or reliable industry results. Based on the science and what’s worked in real-world labs, expect the best performance when keeping bottles well sealed. An airtight amber vial blocks light and limits air, both low-cost but essential measures.
Even indirect sunlight or bright lab lighting causes trouble. Setting bottles on a sunny bench is a rookie mistake. Amber bottles or covering containers with foil shields the chemical from light, preserving that pale appearance scientists expect.
Don’t toss 3-Methoxycatechol into the standard cabinet. Choose a cool space well away from moisture sources and chemical hazards. Refrigerators work if humidity stays low and jars stay tightly closed. Avoid freezers unless a protocol truly calls for it. Extreme cold isn’t always a friend to packaging or contents.
Regular temperature monitoring serves up peace of mind. I had one near-miss as an intern when lab heat failed and a host of chemicals—including 3-Methoxycatechol—sat for days far below their safe range. Some departments invest in digital recorders to alarm if temperatures wander, and I think every lab manager should follow that example for anything this sensitive.
You can’t talk storage without mentioning contamination. Moisture sneaks in on spatulas, gloves, or ambient air. Each exposure introduces the risk of unwanted reactions. Dry, clean tools become a front-line defense. I always tell new researchers: treat your bottle as if it’s yogurt in a college fridge—don’t double-dip.
Desiccators filled with drying agents shield the compound from moisture, an old trick as useful now as when first taught. Proper labeling on every container reinforces safe handling and avoids confusion between lookalike powders.
Some shops run regular inventory checks, logging visual changes. If a chemical’s color shifts, that signals degradation. At my last job, we scheduled product reviews twice a year to weed out anything past its prime, an easy win for lab safety.
Don’t forget safety protocols. Anyone dealing with 3-Methoxycatechol should keep safety data sheets on hand. Proper personal protective gear—gloves, glasses—protects the researcher and guards against trace contamination.
Established guidelines from groups such as Sigma-Aldrich and the Merck Index recommend storing this compound dry, at low temperature, and shielded from direct light. Following these standards limits product loss, supports regulatory compliance, and protects both people and results. Good habits in storage don’t just save money; they keep every experiment on target and reduce waste.
Chemists often talk about purity as if it’s the foundation stones of good work. In my time running reactions and troubleshooting stubborn syntheses, purity played a bigger role than most realize. Take 3-Methoxycatechol – a molecule used in everything from pharmaceuticals research to dye studies. Even the tiniest bit of contamination can send experiment results sideways or leave questions hanging over how safe or reproducible your work will be.
The purity of a reagent like 3-Methoxycatechol speaks directly to the integrity of both the process and the outcome. Anything less than a high grade can lead to problems. Drug researchers, for example, could see impurities muddy their data or introduce unexpected effects. Analysts might miss critical markers on their chromatography runs, or see mystery peaks show up when the sample should be clean.
Manufacturers sell this compound in several grades: technical, laboratory, and high-purity. Technical grades have more of the “other stuff” lingering. Lab grades step it up, targeting fewer impurities. The highest end, sometimes called research-grade or HPLC-pure, sees extra rounds of purification. That top-end product often reaches 98%, 99%, or even higher purity based on analyses like HPLC or NMR. These numbers aren’t just for show—they have real consequences in the lab.
Take a routine prep: using technical-grade material for sensitive biological testing risks introducing unknown confounders. Even for industrial production, high-grade material reduces the chances of expensive clean-up steps later. I once saw a project almost derailed because no one checked the difference between “lab” and “technical.” Only after re-purifying their 3-Methoxycatechol did the team finally see consistent performance.
Labs use different tools to check what’s really in a bottle. HPLC can pull apart the contents and show a clear purity number. NMR and GC-MS fill in other details. Reputable suppliers supply certificates of analysis that spell out their test methods and confirm the grade you’re holding matches what’s promised. A batch marked as 99% really should mean at least 99% pure by that method.
From experience, not all vendors meet the same bar—even when paperwork looks official. In the absence of in-house testing, lab teams have learned to prefer suppliers with long-standing reputations. Stories circulate about corners cut by some vendors looking to boost profits. This kind of risk means new sources always face extra scrutiny before their goods get used in important work.
Researchers and companies alike can address purity headaches by demanding transparency. Ask for batch-specific data. Take time to compare certificates, and, for critical projects, don’t be afraid to test samples independently. Choosing a certified supplier with clear documentation and a track record for reliability saves money and time in the long run.
Any chemist who’s suffered through the effects of impurities doesn’t need convincing of the value of high-purity reagents. It cuts down troubleshooting, boosts reproducibility, and supports research that stands up to scrutiny. In critical fields like pharmaceuticals or environmental science, that’s not just convenience; it’s responsible science.
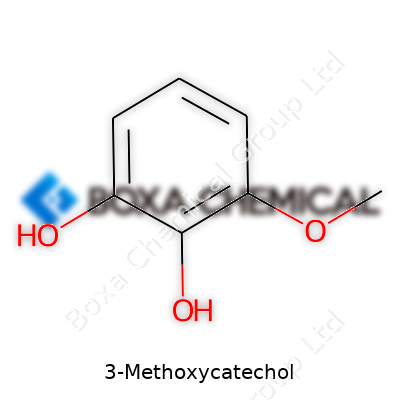
| Names | |
| Preferred IUPAC name | 3-Methoxybenzene-1,2-diol |
| Other names |
3-Hydroxyguaiacol 3-Methoxy-1,2-benzenediol 3-Methoxy pyrocatechol |
| Pronunciation | /ˌθriː.mɪˈθɒk.si.kæt.ɪ.kɒl/ |
| Identifiers | |
| CAS Number | 621-56-7 |
| 3D model (JSmol) | `3Dmol.c('C1=CC(=C(C(=C1)O)OC)O')` |
| Beilstein Reference | 136184 |
| ChEBI | CHEBI:16204 |
| ChEMBL | CHEMBL342941 |
| ChemSpider | 81542 |
| DrugBank | DB04260 |
| ECHA InfoCard | echa.europa.eu/information-on-chemicals/infocards/100.007.362 |
| EC Number | 3.1.1.6 |
| Gmelin Reference | 135168 |
| KEGG | C06500 |
| MeSH | D008770 |
| PubChem CID | 6953 |
| RTECS number | GG9625000 |
| UNII | 1X189A5N9X |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C7H8O3 |
| Molar mass | 154.15 g/mol |
| Appearance | White to light brown crystalline powder |
| Odor | odorless |
| Density | 1.32 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.42 |
| Vapor pressure | 0.0000568 mmHg at 25°C |
| Acidity (pKa) | 9.3 |
| Basicity (pKb) | 10.35 |
| Magnetic susceptibility (χ) | -62.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.578 |
| Viscosity | 1.255 mPa·s (25 °C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 137.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -262.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3662 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | R04CC20 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 113°C |
| Autoignition temperature | 424 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): 730 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2200 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50-200 ppm |