A lot of stories in chemistry begin with trial and error in a lab, but the roots of 3-Heptadecylcatechol reach back to natural products research in the early 1900s. Early chemists looking into plant exudates, especially those from the Anacardiaceae family, tripped over long-tailed catechols as a defense mechanism in nature. Those itching rashes caused by urushiol in poison ivy often come down to molecules like this. Over decades, as extraction and isolation techniques sharpened, researchers gained more clarity on its nuances. Explorations into industrial chemistry in the mid-20th century further highlighted its uses beyond toxicodendron species, driving new methods for controlled synthesis and modification.
3-Heptadecylcatechol features a distinctive pairing: a benzene ring dual-substituted with hydroxyl groups along with a 17-carbon straight alkyl chain. In practical settings, it pops up both in its pure form and in mixtures, sometimes as a minor but active component in broader chemical preparations. Companies shipping advanced fine chemicals regularly include it under specialty catechols. The compound’s structure offers versatility, making it valuable in dozens of projects where hydrophobic interactions and oxidative susceptibility draw interest. Manufacturers, research groups, and industries in coatings, surfactants, and organic synthesis revisit its formulas to fine-tune performance.
If you hold a vial of 3-Heptadecylcatechol in your hand, you’ll probably see a light to pale brown waxy solid, depending on purity. It melts in the moderate range, not as low as wax, not as high as most resins—typically between 60°C to 80°C. The long alkyl tail gives it a greasy texture, barely soluble in water, but readily dissolving in organic solvents like hexane, ether, and chloroform. Its phenolic head loves to oxidize in air, so open containers usually darken over time. On the chemical front, the catechol’s twin hydroxyls ignite reactivity, so it partners in hydrogen bonding, redox reactions, and even polymerization under certain triggers.
Chemists recording batches of 3-Heptadecylcatechol note CAS number 4893-15-8. The molecular weight sits at 432.70 g/mol. Labels flag the compound’s density, usually around 0.95–0.97 g/cm³ at 20°C. Assay grades from reputable suppliers boast greater than 95% purity, with trace metallic or aromatic impurities outlined in the safety sheet. Systems like GHS mark it as hazardous—mild skin irritant, possible respiratory hazard—alerting laboratory staff. Packaging typically uses dark glass bottles, sealed for air and moisture exclusion, with batch number, manufacturing date, and hazard pictograms upfront. Analytical chemists lean hard on NMR, FTIR, and MS data for quality confirmation.
Labs often prepare 3-Heptadecylcatechol through alkylation of catechol with 1-bromopheptadecane. The reaction stirs under inert atmosphere, usually with potassium carbonate as a base and dimethylformamide as solvent. After hours of reflux, technicians filter and wash residues, then purify using column chromatography. For greener aims, some chemists try direct extraction from plant oils, but these are much harder to scale and lack consistent yields. Advanced variations engineer microwave-assisted or catalyzed routes for higher throughput and milder conditions, but most commercial tonnage still anchors on classical alkylation.
3-Heptadecylcatechol rings in as a flexible intermediate. Its catechol group can undergo oxidation by mild oxidants to o-quinones, a trick valuable in crosslinking resins. Standard acylations, sulfonations, or etherifications tweak polarity for specialized tasks. The long heptadecyl tail acts as a hydrophobic anchor, making it useful as a surfactant precursor—combining with polymer chains for controlled self-assembly. Under controlled hydrogenation, the aromatic core sustains modifications without reducing the alkyl tail, adding shy variations to its library. In labs probing anti-corrosive coatings, the molecule often gets blended with epoxy compounds to strengthen humidity and salt resistance.
On a material safety data sheet, 3-Heptadecylcatechol appears under several synomyms, including 1,2-dihydroxy-3-heptadecylbenzene, heptadecylcatechol, and sometimes C17-catechol. Traditional toxicology studies might call out the name "heptadecylresorcinol" incorrectly, though this bears a different structure. Old plant extract literature gives regional names tied to urushiol derivatives. Distributors stick closely to the IUPAC standard for regulatory compliance, preventing mix-ups in downstream supply chains.
Handling 3-Heptadecylcatechol in a lab or plant brings up two challenges: skin irritation and oxidative instability. Gloves and goggles land at the top of every safety checklist. Facilities insist on fume hoods, as even low vapor concentrations can cause lung discomfort over time. Industrial scale operations monitor waste streams for catechol residues due to aquatic toxicity, treating effluent before discharge. Transport regulations force tightly sealed, clearly labeled packaging, with spill kits on-hand to address potential leaks. Training for emergency procedures keeps teams ready, and regular audits track compliance against REACH and OSHA guidelines.
The molecule’s chemical profile sees it called into service across coatings, adhesives, and specialty surfactants. Researchers working on anti-fouling paints for ships reach for catechols with long alkyl chains to repel microbe colonization. Electronics manufacturers explore its binding to metals as a primer for flexible printed circuits. A few niche pharmaceuticals investigate heptadecylcatechol analogs for enzyme inhibition properties, though toxicity limits wide adoption. In analytical chemistry, the molecule helps build calibration standards for urushiol exposure, guiding public health studies in regions rich with allergenic plants.
R&D in this area fights on two fronts: handling and performance improvement. Scientists in coatings constantly seek new ways to marry hydrophobicity with reactive phenolic surfaces, often adjusting the chain length or branching to maximize performance under harsh conditions. Green chemistry teams chase after plant-based sourcing, though purification hurdles still restrict scale. Biotechnology companies try immobilizing enzymes on catechol-modified surfaces, betting on better stability and longer operational life. Environmental health teams dig into the molecule’s interactions in soil and water, modeling breakdown pathways for ecological safety. R&D circles talk about using advanced catalysis, such as C–H activation to add value without harsh side products.
Toxicologists find 3-Heptadecylcatechol difficult to ignore, given its role in allergic reactions from plant contact. Skin contact can lead to inflammation, redness, and, for sensitive individuals, blistering much like poison ivy. Its strong affinity for proteins explains this effect, as the oxidized form binds to skin proteins, triggering an immune response. Inhalation exposure, especially during uncontrolled reactions, can lead to respiratory irritation. Animal studies underline the need for caution—chronic exposure in aquatic environments disrupts certain fish species, prompting careful effluent management. So far, no strong evidence links it to carcinogenicity, but regulatory bodies rank it as a hazardous substance at the workplace.
Looking forward, industries betting on new sustainable materials see some promise in reimagining molecules like 3-Heptadecylcatechol. If researchers refine extraction and purification from renewable sources, costs may fall, opening new roles in eco-friendly coatings or specialty elastomers. Innovations in polymer chemistry could see the catechol group engineered for custom adhesion or anti-microbial surfaces—making medical device coatings, marine paints, or electronics more robust. Toxicity issues keep the pace measured, so green chemistry remains a focus. Real progress depends on balancing chemistry, safety, and sustainability, a challenge that only real-world testing and patient iteration can address.
The name 3-Heptadecylcatechol sounds like it belongs in a high-level chemistry class, not a story about real-world science. Yet, this compound makes an appearance where you’d least expect it. Found primarily in the sap of poison ivy and similar plants, it plays a starring role in those miserable rashes that keep outdoor enthusiasts scratching for days. I’ve fallen victim myself after summer hikes, so this topic isn’t just academic for me.
Most people call it urushiol, a mix of oily compounds found in the Anacardiaceae family of plants. 3-Heptadecylcatechol gives this oil its power. Once it touches skin, the immune system cranks up a defense, and before long red, blistering, and intensely itchy patches appear. The American Academy of Dermatology estimates that 85% of Americans are allergic to poison ivy, oak, or sumac, all thanks to urushiol and compounds like 3-Heptadecylcatechol.
I’ve learned to recognize the “leaves of three,” but not everyone gets that chance. Those who manage parks or run landscaping businesses wind up dealing with skin reactions more often, especially since the oil can stick around on tools, pets, and even firewood for years.
Most of the work around 3-Heptadecylcatechol focuses on understanding allergic reactions at the cellular level. Research published in Clinical Reviews in Allergy & Immunology shows that this compound binds tightly to skin proteins, making it tricky to wash off with just water. Soap and specialized cleansers break down the oil. From a practical standpoint, prevention still gets the best results—wearing gloves and sleeves, and using products designed to remove urushiol before it seeps into the skin.
The medical field studies 3-Heptadecylcatechol for another reason: it’s a perfect model chemical for teaching about contact dermatitis. Doctors test treatments by exposing healthy skin in controlled settings to small amounts of the chemical, then measuring the body’s reaction. This research led to improved approaches for controlling allergic flareups, especially using corticosteroids.
Poison ivy allergies aren’t going away, but education makes a real difference. Simple signs in parks identifying common locations, and wide distribution of pamphlets in outdoor stores help people avoid risky plants. For those working in landscaping, regular health training about urushiol, including 3-Heptadecylcatechol’s role, lowers time lost to rashes and visits to urgent care.
At home, early cleaning works better than home remedies. Commercial washes like Tecnu or Zanfel, developed through years of chemical research including studies with compounds like 3-Heptadecylcatechol, remove the oil before it triggers irritation. These practical tools, paired with public awareness, limit the headache for hikers and workers alike.
Many folks see poison ivy as a minor nuisance. For those concerned about serious infections, or those with severe reactions, learning how chemicals like 3-Heptadecylcatechol operate arms them with knowledge. Protecting skin, using proven cleansers, and spreading word about the science behind the itch shifts the story from fear to practical prevention. Knowledge translates into confidence, and sometimes, a less itchy summer.
Powerful chemicals draw both respect and caution. 3-Heptadecylcatechol, a long-chain catechol compound, stands as no exception. Tackling this material means taking steps to look after both people and the working environment. My years in the lab have taught me that small lapses around catechols can carry outsized consequences.
Before opening a container, hands need decent gloves. Nitrile or neoprene gloves stand up better against catechols than most. Lab coats or dedicated overalls stop direct skin contact, and safety goggles shield eyes against any accidental splashes. I've seen overlooked details—like an untied lab coat—lead to skin rashes from trace exposure. Change gloves and coats if a spill happens. It's tempting to just wipe and keep going, but that small decision comes back to haunt you.
Good ventilation keeps the air clear. Chemical fumes, even from something that looks harmless, travel quietly. Fume hoods serve as more than fancy equipment—they pull vapors away at the source. Years ago, I worked in a cramped lab without proper airflow, and the headaches and eye irritation became a running complaint. Once the fume hood ran reliably, those problems vanished.
3-Heptadecylcatechol asks for cool, dry storage. Direct sunlight or warm temperatures can change its properties and trigger unwanted reactions. Keep containers tightly sealed, ideally with labeling that doesn’t rub off easily. If you see discoloration or leaks, move the container to a safe place and consult with the safety officer right away.
Nobody wants a mess, but accidents do happen. Absorb small spills with dedicated inert absorbents, such as vermiculite. Leave household towels or tissues in the drawer—standard materials won’t contain the chemical effectively. After soaking up the spill, seal everything—used gloves, wipes, absorbent—in heavy-duty disposal bags. Make sure to report the spill. It’s tempting to keep small accidents quiet, but staying honest helps everyone.
Some catechols bite back harder than others. 3-Heptadecylcatechol can irritate skin, eyes, and lungs. Prolonged skin contact sometimes leads to blistering or long-term skin changes. In my own work, someone caught a splash in the eye and learned quickly how painful it can be—eye washing stations matter. If exposure happens, immediate rinsing and medical attention make a difference. Know the location of showers and eyewash units before starting work.
Training shapes habits that last. Anytime a new person joins the team, walk through the handling steps and show where everything is. Habits grow from repetition, so run through emergency drills, not just once but on a regular schedule. I’ve seen emergencies handled calmly because everyone knew what to grab and where to go.
Chemicals like 3-Heptadecylcatechol demand respect. Trust in strict protocols leads to safer work and healthier outcomes. Rushing or cutting corners only creates risk. Take the time to gear up, double-check labels, and support a culture where speaking up about safety feels normal.
3-Heptadecylcatechol might sound complex, but it’s built from two familiar parts: a catechol ring and a long-chain alkyl group. Catechol forms the core — it's a simple benzene ring carrying two hydroxyl groups. Those two hydroxyl groups sit next to each other, locked into the first and second positions on the ring. It gives the molecule sticky hands for chemistry, making it highly reactive and interesting to organic chemists.
The “3-heptadecyl” portion lays out a straight 17-carbon chain. This isn’t some random choice; long hydrocarbon chains matter in biology and technology. They often show up in natural products, surfactants, and biological membranes, influencing how molecules interact with fats, oils, and cell surfaces. In 3-heptadecylcatechol, this chain branches off from the third carbon of that catechol ring. Now, you have a structure that’s part water-loving (those hydroxyl groups) and part oil-friendly (the long hydrocarbon tail).
Take a plain benzene ring and label its six carbons. Attach two hydroxyl groups on carbons one and two; that forms catechol. From the third carbon, extend a straight, unbranched chain of seventeen carbons. That’s the heptadecyl group — no rings, no oddities, just a saturated chain like you’d find in some fatty acids. Put it all together, following IUPAC naming, and you get 3-heptadecylbenzene-1,2-diol.
Long alkylcatechols aren’t just academic curiosities. In my lab days, I handled molecules like this working with plant resins and natural adhesives. These compounds pop up in the secretions of some insect species, a class known as “urushiols.” Poison ivy, for instance, makes a similar molecule — that's where the resin gets its infamously irritating effect. The mix of a sticky, reactive ring and a greasy tail lets it wedge into cell membranes and trigger immune responses. Once I spilled a dilute solution of an urushiol analog on my glove; nothing happened at the time, but a day later, red inflamed skin broke out. Even at small concentrations, their chemistry is potent.
On the other hand, these molecules have uses beyond poison ivy. Their unique structure makes them great at interacting with both water and oils. This dual nature shows up in the formulation of adhesives, coatings, and even in some forms of specialized surfactants. Nature figured out how to make stable, waterproof coatings long before humans did — traditional lacquerware from Japan, for example, uses urushiol-rich sap for its resilience and shine.
Working with 3-heptadecylcatechol, or similar compounds, calls for respect. The same reactivity that lets the molecule stick to everything also makes it tricky to handle safely. Managing exposure, using the right gloves and hoods, and thorough cleanup after experiments kept our lab incidents rare. But these safety steps shouldn’t scare off creativity; careful handling opens up avenues for new materials — waterproof coatings, strong adhesives, and environmentally-responsive surfaces.
Science makes plenty of advances borrowing from nature's molecular toolkit. By understanding classic structures like 3-heptadecylcatechol, researchers don’t just reverse engineer plant defense systems. They also get new ideas for biomimetic chemistry, practical materials, and treatments that interact at the boundary between organic chemistry and biology. Curiosity and good technique uncover real value locked inside these seemingly simple ring-and-chain molecules.
Standing in a chemistry lab, the first thing that grabs your attention is how every bottle has its spot—this isn’t just for neatness. Chemicals like 3-Heptadecylcatechol carry risks. People often overlook the details, but storing this compound well makes a difference. Too many times I’ve seen chemical cabinets crammed, labels missing, caps loose. All it takes is a spill or some unexpected heat from a window for trouble to start.
3-Heptadecylcatechol isn’t as famous as sulfuric acid, but its hazards are real. It can react with air and moisture, and it shouldn’t touch bare skin. Keeping it under control calls for attention to temperature, light, and air exposure.
Never stack this kind of chemical near the sun or any heating equipment. Excess heat ages the chemical faster and can force pressure inside the container. This isn’t just theory—I’ve seen lab glass shatter during summer because someone ignored a sunny window. Place bottles in cool, dark storage—that means a chemical fridge or a low-shelf cabinet. Safety experts recommend temperatures between 2°C and 8°C for sensitive organics; I’ve had similar luck keeping compounds stable inside refrigerators set for biological samples.
Exposure to air and moisture spoils the structure of this catechol. Don’t just twist the cap on tight and assume it’s done. Always use airtight containers, preferably with a gasketed seal. For small quantities, glass vials with Teflon-lined caps work well. Some labs go an extra mile, storing sensitive chemicals under dry nitrogen or argon to keep oxygen and water away. Dry boxes and desiccators can hold backup stocks—these work better than placing the container in a desk drawer and hoping for the best.
A label might sound basic, but it saves lab partners from guessing games. Write a full date, concentration, hazard class, and the exact chemical name. During frantic days, the small habit of precise labeling saves everyone from confusion, and it gives emergency crews a fighting chance during accidents.
Whenever you open the bottle, gloves and safety goggles matter. Even a faint whiff off the rim can irritate. Always work with 3-Heptadecylcatechol in a working fume hood. Spill kits need to be on hand before you open the bottle—never after. From experience, mopping up with paper towels is useless. Absorbent pads and a plan for toxic-waste disposal make any cleanup less dangerous.
A well-ventilated storage area makes a difference in emergencies. Fume accumulation happens quietly, especially overnight. A small vent fan or regular air checks can prevent headaches and more serious injuries.
Proper storage isn’t only for researchers. Many chemical accidents happen in industries and schools that cut corners. Training people is just as important as buying the right cabinet. Take the extra minute to double-check—rushed jobs lead to leaks, contamination, and ruined experiments. That’s costly in money as well as health.
Chemicals like 3-Heptadecylcatechol test the discipline of a workplace. Sanitation, training, and upkeep turn an ordinary storeroom into a safe space. Storing this compound right isn’t only about following rules; it turns everyday work from risky to routine, protecting skin, air, and the whole community.
3-Heptadecylcatechol doesn’t show up in everyday conversation. This long-chained catechol derivative has found its way into some specialized industrial and research settings. I once worked in a lab where the walls steadily collected warning labels for substances just like this. Safety sheets carried complex words, but at their core, you recognized the old message: respect the risks or pay a price with your health.
Available toxicological data isn’t as thick as an encyclopedia, yet researchers have observed some real dangers. Catechols as a chemical group cause trouble in the human body. Many catechols trigger nasty skin reactions. Workers exposed to their fumes or dust complain of rashes, swelling, and unexpected redness in places that brushed against lab benches or factory railings. Skin doesn’t just look red; it turns raw. People report blisters and persistent itching. Long exposure tends to pile up the damage—dry, cracked skin opens the door for infection.
Breathing in the stuff isn’t harmless, either. Dust and fine particles of chemicals like 3-Heptadecylcatechol irritate the nose and lungs. Regular contact can set off asthma-like symptoms. It gets hard to ignore the tight chest and cough that lingers long after you leave the room. Even handling the chemical with gloves sometimes stirs up volatile fumes that drift into nearby air. That sting in your sinuses and lungs signals that your body senses a threat.
Industrial safety guidelines treat catechols as potential carcinogens. There’s enough evidence from related molecules—take urushiol, the irritant in poison ivy—that makes you think twice about brushing against a beaker. Long-term studies draw lines between chronic exposure and increased tumor risk. Cells under constant attack often mutate. As these chemical changes build, so does the cancer risk down the road.
The liver faces another burden. Studies on extended exposure suggest liver enzymes kick into overdrive, trying to process and flush out these compounds. Over time, the effort strains your liver’s filtering power. Accumulated stress can lead to chronic inflammation or even tissue scarring. If you’ve ever known anyone with liver trouble, you know that even daily routines—diet, energy levels, alcohol tolerance—shift in frustrating ways.
Good airflow keeps fumes moving out of the workspace. Proper personal protective equipment, especially gloves and well-fitted respirators, plays a key role. I remember double-gloving for certain solvents that seemed to slice through latex barriers. For substances like 3-Heptadecylcatechol, chemical-resistant gloves backed up by face shields or goggles offer much better odds.
Training matters, too. A new hire receives more than a list of rules; the older hands teach the feel of safe handling—when a glove fits wrong or when a fume hood works at half-power. Keeping access restricted, using sealed containers, and scheduling regular health check-ups all serve as early warning measures. Medical monitoring catches suspicious changes before they spiral into bigger problems.
3-Heptadecylcatechol isn’t just a hard-to-pronounce chemical; it brings significant health risks for those touching or inhaling it at work. Physical reactions can pop up fast, with bigger problems lurking after years of exposure. Rigid safety habits, top-tier protective equipment, and regular health checks together keep these risks in check.
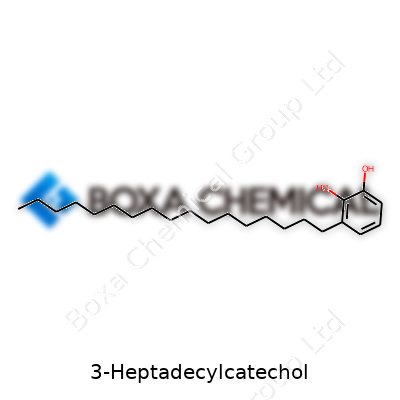
| Names | |
| Preferred IUPAC name | 3-heptadecylbenzene-1,2-diol |
| Other names |
Urushiol |
| Pronunciation | /ˌθriː hɛp.təˈdɛs.ɪl.kəˈtiː.kɒl/ |
| Identifiers | |
| CAS Number | 16842-52-1 |
| Beilstein Reference | 2045958 |
| ChEBI | CHEBI:139592 |
| ChEMBL | CHEMBL3424926 |
| ChemSpider | 26734311 |
| DrugBank | DB14095 |
| ECHA InfoCard | ECHA InfoCard: 100_029_791 |
| EC Number | 205-267-6 |
| Gmelin Reference | 716551 |
| KEGG | C16535 |
| MeSH | D017862 |
| PubChem CID | 122242 |
| RTECS number | SKJ87J4W2Y |
| UNII | 52PL86YK5C |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C23H40O2 |
| Molar mass | 422.70 g/mol |
| Appearance | White to gray powder |
| Odor | Odorless |
| Density | 0.899 g/cm3 |
| Solubility in water | insoluble |
| log P | 8.72 |
| Vapor pressure | 6.58E-09 mmHg |
| Acidity (pKa) | 10.30 |
| Basicity (pKb) | 13.46 |
| Refractive index (nD) | 1.577 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.4177 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 376.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –430.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -10591.5 kJ/mol |
| Pharmacology | |
| ATC code | D11AX04 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08, GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H317, H319, H411 |
| Precautionary statements | P280, P302+P352, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 182 °C |
| LD50 (median dose) | LD50: 40 mg/kg |
| NIOSH | SN29700 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Heptadecylcatechol: "No specific OSHA PEL established |
| REL (Recommended) | 0.02 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
3-Heptadecylcatechol Cardanol 2-Methylcardol 5-(Pentadecyl)resorcinol |