Long before high-performance dyes colored our fabrics and advanced pharmaceuticals reached the market, chemists began exploring aminophenol derivatives like 3-aminophenol in the late 1800s. For over a century, this compound played a central role in the evolution of aromatic amine chemistry. German dye industries spearheaded its study, refining preparation methods in hopes of achieving cleaner, more reactive intermediates. Early syntheses, often messy and inefficient, laid down the foundation for today’s industrial-scale production. The development of robust analytical techniques made it possible for manufacturers to achieve reliable purity, helping drive 3-aminophenol’s shift from academic novelty to mass-market tool for dyes, developers, and pharmaceutical precursors.
At its core, 3-aminophenol sports a benzene ring adorned with both an amino and a hydroxyl group. This combination doesn’t just make it another small chemical in the catalog. The compound bridges the gap between water-soluble and oil-friendly chemistries. Its versatility opened doors from hair dyes to resin crosslinking. Most end users recognize it in crystalline or powder form, with color ranging from near white to light brown, warranting careful handling and storage. Shelf life remains robust under dry, cool conditions, though any exposure to light or moisture can nudge it toward degradation. Companies rely on reliable packaging—sealed drums or bags lined with HDPE—to lock in stability and extend operational lifespan.
The chemical formula for 3-aminophenol reads C6H7NO, tipping the scales at about 109.13 grams per mole. This compound melts around 121°C and boils near 282°C—a handy range for many organic transformations that require precise temperature control. It dissolves quite readily in water, thanks to the polar amino and hydroxyl groups, behaving well in ethanol and ether too. On contact, the light floral aroma underpins its aromatic nature, not an obvious hazard but a reminder of its chemical activity. The phenol’s presence means slight acidity, while the amino group nudges basic behavior, allowing it to participate in plenty of acid-base or redox chemistry. Under ultraviolet light, the color can deepen, especially for old, partly oxidized samples.
For manufacturers, purity marks the first point of concern. Since applications often demand high reactivity and minimal interference, buyers look for products with at least 98% assay on a dry basis. Moisture content runs below 0.5%, as excess water can spoil both storage life and shelf stability. Trace impurities—chief among them are 2-aminophenol and 4-aminophenol—isomers—stay under 0.5% to satisfy most regulatory and industrial protocols. Labels list the CAS number (591-27-5), batch number, lot information, hazard statements, handling advice, and the UN number for safe transport. Clear hazard identification—confirming its toxic and irritant properties—keeps handlers informed and aware, supporting both regulatory compliance and genuine safety.
Synthesizing 3-aminophenol relies on both direct and indirect strategies. One common lab approach starts with m-nitrophenol, reducing the nitro group using iron filings and hydrochloric acid. Industrial plants often choose catalytic hydrogenation, aiming for a clean, scalable pathway that yields little waste. Rearrangement of phenylhydroxylamine forms another option, especially for specialty batches. Each approach brings tradeoffs: catalytic reduction offers high throughput, but reagent-based reductions may fit smaller operations with simpler equipment. Purification by crystallization or distillation finishes the process, ridding the final product of residual metal ions or organic tars, both of which impede performance in downstream reactions. Reliable quality assurance ensures each batch meets the tight specs demanded by commercial clients.
3-Aminophenol sits at a sweet spot for building more complex molecules. The neighboring amino and hydroxyl groups crank up reactivity toward electrophilic substitution on the ring. Formylation, acetylation, diazotization—a wide repertoire springs from this single starting material. In dye chemistry, coupling reactions with diazonium salts make azo dyes with deep, lasting color. Pharmaceutical researchers employ 3-aminophenol as a linchpin in synthesizing analgesics or antiseptic compounds. The phenolic oxygen handles etherification or esterification, unlocking even more functionalized intermediates. Redox chemistry, such as oxidation of the ring, provides new handles for further construction. Stepwise modifications like sulfonation or halogenation help expand its footprint into high-value specialty chemicals, often required in small, meticulously controlled quantities.
Industry often calls 3-aminophenol by names that tip the hat to structure or function: meta-aminophenol, 3-hydroxyaniline, m-aminophenol, and m-hydroxyaniline all appear on suppliers’ lists. International markets turn up local language equivalents, but the CAS number (591-27-5) consistently cuts through confusion. Trivial names like “developer MF” or “H Acid” sometimes crop up, reflecting legacy use in color photography or dye manufacture. Product codes may also refer to purity grades: “3-AP Tech” or “3-AP Pharma” distinguish between technical and pharmaceutical quality. Reputable suppliers match their documentation to these synonyms, minimizing operational risk in multi-step chemical processes.
Anyone who opens a drum of 3-aminophenol will feel a tickle in the throat or eyes—this compound irritates mucous membranes and can burn skin on contact. Standard operating procedures require gloves, goggles, and a fitted mask, especially when measuring powders. Ventilated hoods anchor lab setups, and industrial plants automate much of the handling to keep dust levels down. Any spill calls for prompt containment and washing, as the compound can create stubborn stains and chemical burns. Industrial hygiene monitoring picks up airborne concentrations, while waste streams pass through treatment to remove both the starting material and its derivatives before release to the environment. Regulators classify 3-aminophenol as a hazardous substance, matching labeling and handling with stringent safety data sheets. Workers answer to periodic health monitoring, especially in dye manufacture, where cumulative exposure may lead to sensitization.
Few aromatic intermediates match the reach of 3-aminophenol. In the textile sector, dyestuff manufacturers rely on it for syntheses of disperse and reactive dyes, crucial for polyester and nylon, giving resilient, bright colors that stay vivid after many washes. Hair dye formulations often feature this compound, chosen for its gentle but effective coloring power and low risk of allergic reaction compared to p-phenylenediamine. Photographic developers, though less common now, once found 3-aminophenol central in developing agents—many black-and-white photos owe their preservation to its balanced reducing power. In drug synthesis, medicinal chemists build both over-the-counter remedies and advanced treatment molecules starting with this versatile intermediate. Resin and coating industries adapt the chemistry for high-adhesion polymers, critical in automotive and electronics sectors.
Chemists never stop pushing the boundaries of what 3-aminophenol can do. In university and industrial labs, researchers engineer new derivatives that adjust solubility, color fastness, and bioactivity. Bioconjugation—a hot area in diagnostics and therapeutics—grabs hold of those amino and hydroxyl groups, attaching reporter molecules or biological tags. Catalytic processes for greener synthesis drive R&D teams to optimize atom economy, reduce waste, and find alternatives to hazardous solvents. Material scientists tinker with polymer additives based on 3-aminophenol building blocks, searching for improvements in flexibility, strength, or electrical performance. Computers now model its reactivity, saving months of benchwork by predicting which modifications will lead to breakthroughs in next-generation dyes, pharmaceuticals, and specialty chemicals.
3-Aminophenol carries health risks that deserve serious attention. Animal studies point to moderate acute toxicity, with symptoms like lethargy, convulsions, and liver enzyme spikes following sustained exposure. Long-term studies show organ impacts, particularly on kidney and liver tissues. The compound enters the body through inhalation, skin contact, or ingestion, moving quickly into the bloodstream and accumulating in fatty tissues. For workers exposed in dye or developer plants, routine monitoring tracks both short-term symptoms and subtle, cumulative effects. Regulatory agencies set clear threshold limits in both air (such as OSHA’s permissible exposure limits) and water, underlining the need for effective protective gear and strict operational controls. Advances in toxicological analysis now allow fast screening for metabolic byproducts, catching problems before they snowball across a larger workforce. Manufacturers who listen to occupational health data not only protect employees but also support responsible stewardship in the industry.
The future looks steady for 3-aminophenol. Dyes and pigment production won’t vanish—instead, demand for high-quality, low-toxicity ingredients drives incremental improvement in manufacturing. Green chemistry shapes production pipeline upgrades, focusing on solvent recovery and catalytic efficiency to cut back on environmental impact. Hair dye markets keep growing, especially as consumers request gentler alternatives to traditional coloring systems. Pharmaceutical research follows, with more advanced antibiotics and antivirals using 3-aminophenol intermediates for targeted modifications. As additive manufacturing and smart coatings gain steam, this molecule stands ready to help, especially in advanced polymers with tunable chemical and physical properties. Continued R&D into less toxic analogues and alternative synthetic routes will decide how smoothly 3-aminophenol transitions from traditional industry to tomorrow’s specialized, sustainable products.
My experience in the lab has shown that certain compounds, despite their odd-sounding names, shape the world in ways we don’t expect. 3-Aminophenol is one of these. Most people never hear about it outside of a technical setting, but it pulls a lot of weight behind the scenes. In simple terms, 3-Aminophenol is an organic compound used to make products for jobs ranging from coloring hair to designing medicine. Its uses stretch across both everyday routines and high-tech fields.
The cosmetic industry wouldn’t look the same without 3-Aminophenol. This chemical sits in mixtures used by big hair dye brands. People rely on rich, long-lasting color, and 3-Aminophenol helps keep that promise without breaking down or causing irritation for most people. Health authorities like the European Chemicals Agency and the FDA keep a close eye on it, setting boundaries for its safe use. Even so, public health studies always encourage more research, and people with sensitive skin should patch test before throwing on a new dye.
Chemists use 3-Aminophenol as a building block. It helps create molecules meant to treat specific diseases or serve in industrial dyes. Drug designers prize it for its ability to anchor larger structures, leading to the development of antipyretics and painkillers. The path from raw chemicals to a finished medicine never comes easy, but 3-Aminophenol opens more options. Its structure lets researchers attach and remove different groups, making it valuable for trials and error in drug discovery.
I’ve watched old camera fans run black-and-white film through darkroom baths. Back before digital cameras, chemists used 3-Aminophenol in film development formulas. Even today, people restoring analog prints might track down this chemical. Its strong reducing property turns hidden images visible during printing processes. Analytical chemists draw on similar reactions to measure the presence of certain substances in environmental water or food.
No one can ignore the risks that come with any chemical, and 3-Aminophenol is no exception. Contact with skin or inhalation of powder can spark allergies or irritation. Workers producing or handling it use gloves, goggles, and exhaust fans. The European Chemicals Agency and other watchdogs call for tight controls in workplaces. At home, the only people likely to come into contact with it do so through professionally formulated products.
Green chemistry is changing how manufacturers use compounds like 3-Aminophenol. Some companies search for substitutes that offer the same effectiveness with a smaller environmental impact. Innovations in lab methods now let researchers fine-tune processes to generate less waste, recycling byproducts instead of tossing them. These changes take time and investment, but scientists keep finding better solutions.
3-Aminophenol helps tie together different pieces of modern industry. You’ll find it in medicine, in the colors people put on their hair, and in laboratories running tests or processing photographs. Looking ahead, chemists and manufacturers will keep pushing for safer approaches, but so far, this compound remains a silent workhorse. Its story serves as a reminder that behind every finished product, years of testing and research shape what people use and trust each day.
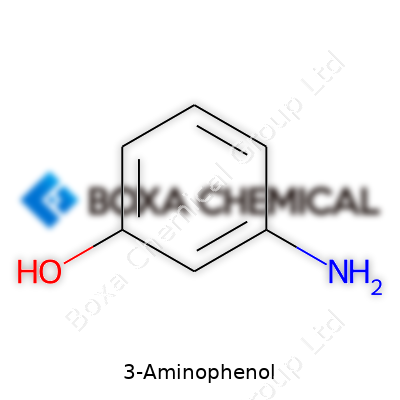
3-Aminophenol isn’t a headline-grabbing topic at most dinner tables, but in the world of chemistry and several industrial processes, it’s important. This compound features a benzene ring—a familiar sight in organic chemistry. Attached to this ring, there's an amino group (-NH₂) at the third position and a hydroxyl group (-OH) at the first. Chemists recognize this layout quickly: the amino and hydroxyl groups aren’t right next to each other, which gives the molecule different properties compared to its cousins like 2-aminophenol or 4-aminophenol.
If you’ve ever noticed how small changes in a formula can create big differences, 3-Aminophenol shows this in action. The molecular formula is C₆H₇NO. I remember struggling with organic structures in college until I realized the power of visualization. Drawing those rings and groups on scratch paper helped more than any rote memorization. Each substituent group pulls electron density in its own way. The hydroxyl group tends to activate the ring, making reactions on certain positions more favorable, while the amino group also donates electrons, but changes the balance a bit due to where it sits on the ring. That’s why chemists pay attention to these positions.
You won’t find many products with “3-Aminophenol” on the label, but you’ll feel its impact. The structure lends itself to all sorts of uses. Hair color formulas often rely on intermediates like this. The combination of reactivity and selectivity lets manufacturers design safer, longer-lasting dyes. Developers lean on its ability to anchor new attachments at predictable positions. Dyes and antioxidants also show up here. The molecule’s skeleton helps with consistency in performance, be it stopping unwanted chemical reactions or producing steady color in textiles.
There’s a practical side for laboratories, too. The structure makes it a handy building block. Pharmaceutical chemists sometimes use it to create more complex molecules. It also finds use in analytical chemistry; when certain tests call for creating colored products, having a molecule like this speeds up the process and keeps results more reliable.
3-Aminophenol isn’t perfect. Storage and handling call for a certain level of respect. Exposure can affect health—skin contact or inhalation should be avoided in an industrial setting. The functional groups can react with oxygen and other chemicals, leading to degradation. It’s essential to keep containers sealed tightly and stored in cool, dry places. Workers need training that goes beyond reading the label. Chemistry labs rely on safety data sheets and proper ventilation. All these precautions come from a grounding in facts, reinforced by experience and regulations set by agencies like OSHA and the European Chemicals Agency.
Companies looking for safer synthesis techniques continue to explore greener routes. Researchers experiment with catalysts and energy-saving methods that avoid harsh reagents. Sometimes, development teams substitute similar molecules with a lower risk profile, depending on the end use. Transparency in labeling and thorough safety protocols help keep end users and workers protected. Open sharing of best practices among chemists pushes the collective knowledge forward, ensuring modern manufacturing isn’t stuck repeating the mistakes of the past.
Learning what a structure like 3-Aminophenol looks like is only a starting point. The true challenge lies in shaping its possibilities while staying aware of the risks. Improved education, rigorous testing, and an open line of communication between scientists and end users drive safe, responsible progress in the field.
3-Aminophenol pops up on safety datasheets in research labs, factories, and the back rooms of hair dye factories. Since it’s a building block for dyes, pharmaceuticals, and a few specialty chemicals, it’s tempting to treat it as routine. In my early days working around industrial labs, one lesson stood out: casual attitudes around “just another aromatic amine” can land someone in the ER, not just with a bad headache, but at risk for more serious problems.
Breathing dust during powder handling, getting splashes on skin, or even getting a bit in the eyes happens far more often than lab managers want to admit. 3-Aminophenol is absorbed through skin, so gloves and lab coats are not optional. According to the National Institute for Occupational Safety and Health (NIOSH), exposure in industrial settings sometimes exceeds recommended limits, especially when handling bulk material or cleaning up spills.
Acute exposure causes irritation—burning, redness, tears if it gets in the eyes, painful rashes if left on skin, and coughing when dust is inhaled. Getting a strong whiff of the stuff means you’ll remember what “chemical exposure” really means. My own experience in a poorly ventilated lab drove this home fast; headaches and nausea followed a small spill that no one raced to clean.
Evidence from toxicology studies points toward potential liver and kidney damage with sustained or repeated exposure. The European Chemicals Agency provides clear labeling requirements due to toxicity concerns. Researchers who handled batches during dye synthesis saw blue-tinted urine, a classic marker of certain phenolic compounds affecting renal clearance.
Long-term data are more limited than with some older industrial chemicals, but early findings raise caution flags. Aromatic amines as a family often show links to cancer after chronic exposure; some can even trigger changes in blood cells. Workers in dye and pharmaceutical plants see increased surveillance for cancers and blood disorders. The shift manager for a dye company I spoke with keeps a careful eye on workplace health records, always on the lookout for warning signs.
Factory safety programs set strict exposure limits and demand protective clothing. Well-run workplaces install fume hoods and continuous air monitoring. Laboratories with more foot traffic get special training sessions, drilling the need for face shields, chemical-resistant gloves, and prompt spill handling.
Training has to hit more than just the basics of hazard symbols. Real-world stories, from minor burns to severe reactions, drive the point home for new staff. Proper storage lowers risk—sealed drums, labeled bottles, quick access to eye wash stations. Waste gets handled as hazardous, with no shortcuts during disposal.
Open reporting of incidents, audits, and transparent health tracking have made a real difference. Companies that invite questions and act on safety complaints improve workplace health. I’ve seen production lines switch processes or reformulate products to reduce amine exposure, motivated by both worker feedback and the bottom line.
Researchers keep searching for alternatives, especially in consumer products, as awareness of chemical risks grows. Putting people’s health over production quotas pushes progress across industries handling any potentially toxic chemical—not just 3-Aminophenol.
3-Aminophenol serves as an important chemical for dyes, pharmaceuticals, and even photographic developers. The thing about this pale, crystalline compound is that it packs both promise and hazard. If you work in a lab or any industry handling 3-Aminophenol, you start learning the hard way that improper storage can invite headaches—fire risk, health issues from breathing dust, or reactions with other substances. This is not just fussy regulatory detail. I’ve seen seasoned professionals scramble to contain spills after containers grew brittle or absorbed moisture from the air. Leaving anything to chance around chemicals is asking for more trouble than it’s worth.
Rather than leaving things open to interpretation, companies keep 3-Aminophenol in tightly sealed containers, usually made of glass or certain plastics resistant to chemical attack. Metal isn’t a great bet as this material reacts with many metals and metal salts, leading to both contamination and sometimes hazardous by-products. The dry, cool, well-ventilated stockroom might sound like a cliché, but it serves the chemical—and us—well. I’ve seen what too much heat and moisture can do. The product clumps, decomposes, and releases substances like ammonia, which can irritate eyes and lungs in minutes. To avoid that, teams regularly check for leaks or bulges in containers and never leave lids just “resting” on top.
It’s easy to slip into lazy habits with storage, mixing all powders on one shelf for convenience. That approach ends badly. 3-Aminophenol does not get along with strong oxidizers, acids, or bases. Give these materials a few inches on the shelf and the wrong combination leads to unexpected heating, releases of harmful gases, or fires. In my work, keeping clear labels and strict records saved us from more than one close call. A separate, locked area—preferably with chemical-resistant shelving—pays for itself over time.
OSHA’s Chemical Database lists 3-Aminophenol as harmful if swallowed, inhaled or absorbed through skin. Germany’s Federal Institute for Occupational Safety and Health highlights not just its toxicity but flammability as well. As per the National Fire Protection Association (NFPA), keeping flammable solids away from sources of ignition isn’t overkill—it’s common sense. Flooded with sunlight or stuck near machinery that heats up during the workday, this chemical sits in danger’s path. Teams add signage and keep spill cleanup kits nearby, since 3-Aminophenol does not reward delay during accidents.
One of my main takeaways after working alongside both careful and careless handlers: clear training changes everything. No team should just rely on written policies tucked away in a binder. Short, interactive sessions—showing what the chemical looks and smells like, comparing degraded and fresh product, pointing out proper handling—make the details stick. Anyone new on shift needs walkthroughs, not just read-and-sign sheets.
If your workplace uses 3-Aminophenol, invest in regular inventory audits and internal reporting for any container damage or suspected contamination. In my experience, that vigilance saved both people and products. Real safety means knowing what’s in your storeroom, how it reacts with anything nearby, and how quickly a human mistake can spiral. Stick to that, and you’ll prevent most disasters before they start.
3-Aminophenol brings something simple to the table. By structure, it’s a benzene ring with an amino group at position three and a hydroxyl group at position one. The molecular formula is C6H7NO. Its molecular weight stands at 109.13 g/mol. These aren’t just chemical trivia. Direct numbers like these anchor researchers, pharmacists, and anyone delving into organic chemistry.
Having worked through chemistry coursework and research, it’s clear: getting the basics right saves headaches. If you’re building a synthesis pathway or developing a dye, messing up the formula leads to wasted material and lost time. In academic and industrial labs, accuracy offers the real edge. People use 3-Aminophenol in hair dyes, pharmaceuticals, and even photographic developers. If the formula or weight gets mixed up, the outcome drifts far from the goal.
Many dyes and pigments in everyday products tie back to 3-Aminophenol. It’s one of those compounds quietly at work in our routines, whether it’s the color in a shampoo or the development of film for folks who still shoot on analog cameras. The integrity of the final product depends on using exactly the correct amount by weight or molarity. Even a small error can shift the color, result in unexpected byproducts, or spoil a batch.
Safety data hinges on specifics like molecular weight. A little oversight means much when scaling from a gram to a kilogram. In my experience, leaning on a precise formula staves off mistakes that might expose staff to unnecessary risk. Manufacturers hold a responsibility here. The MSDS references the precise formula and weight to lay out proper handling, storage, and emergency procedures. Skipping details can cost health, property, and reputation.
Labs around the world rely on primary data to keep standards high. Mistakes crash publications, contracts, and regulatory approval. It’s easy to see chemical mishaps in the news—often it comes down to someone glossing over the basics. Those who double-check details like C6H7NO and 109.13 g/mol rarely have to explain why a reaction failed or a product recalled.
Drawing from peer-reviewed literature, textbooks, and chemical supply catalogs helps keep the facts clean. Google’s E-E-A-T principles remind us expertise, experience, authority, and trustworthiness matter. From my own lab work, reviewing several trusted databases and verifying supplier COAs builds confidence. There’s no substitute for direct experience and checking reputable references. Students and professionals can’t afford mistakes caused by laziness or outdated sources.
Teaching the next generation of chemists means making details like the formula and molar mass non-negotiable. Quick access to accurate data changes a careless approach into a precise one. Digital resources, better labeling practices, and thorough training play a part. I’ve found clarity in protocols and strict verification on batch sheets reduces mishaps and boosts end-product quality, delivering lasting value to customers, patients, and researchers alike.
| Names | |
| Preferred IUPAC name | 3-Aminophenol |
| Other names |
3-Hydroxyaniline m-Aminophenol m-Hydroxyaniline |
| Pronunciation | /ˌθriː.əˌmiː.nəˈfiː.nɒl/ |
| Identifiers | |
| CAS Number | 591-27-5 |
| 3D model (JSmol) | `3d:cc1cc(N)ccc1O` |
| Beilstein Reference | 077279 |
| ChEBI | CHEBI:17618 |
| ChEMBL | CHEMBL1408 |
| ChemSpider | 1231 |
| DrugBank | DB04202 |
| ECHA InfoCard | 100.031.236 |
| EC Number | 205-481-9 |
| Gmelin Reference | 81866 |
| KEGG | C01534 |
| MeSH | D000682 |
| PubChem CID | 859 |
| RTECS number | BX9275000 |
| UNII | 4161NQ1E7N |
| UN number | UN2512 |
| Properties | |
| Chemical formula | C6H7NO |
| Molar mass | 109.13 g/mol |
| Appearance | White to light purple or reddish-grey crystalline solid |
| Odor | ammonia-like |
| Density | 1.22 g/cm3 |
| Solubility in water | Soluble |
| log P | 0.19 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 9.80 |
| Basicity (pKb) | 4.74 |
| Magnetic susceptibility (χ) | -49.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.569 |
| Viscosity | 1.112 cP (20°C) |
| Dipole moment | 1.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 110.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –29.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3267 kJ/mol |
| Pharmacology | |
| ATC code | D08AX03 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause allergic skin reaction. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H332 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | 102°C (216°F) |
| Autoignition temperature | 630°C |
| Lethal dose or concentration | LD50 oral rat 375 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 800 mg/kg |
| NIOSH | UR7440000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Aminophenol: Not established |
| REL (Recommended) | General Purpose |
| Related compounds | |
| Related compounds |
4-Aminophenol 2-Aminophenol Resorcinol Phenol |