The story of 3,5-xylenol stretches back over a century. Chemists in the late nineteenth and early twentieth centuries looked for new phenolic compounds to expand industrial chemistry and medicine. As part of that search, they isolated and examined different methylated phenols coming from coal tar and crude oil fractions. Early research found 3,5-xylenol reliable for its robust antiseptic properties. It took substantial trial and error to nail down synthesis routes. Over time, refinements in alkylation of phenol and extraction from mixed xylenol isomers reduced impurities and slashed production costs. The process stayed largely industrial because consumer products needed better safety measures before rollout. Later decades saw pharmaceutical and agricultural interests take greater notice, especially as chemical factories got better at separating isomers for research and large-scale manufacturing.
3,5-Xylenol, also called 3,5-dimethylphenol, stands among the most trusted phenolic antiseptics. Its popularity isn’t just about killing germs. Industrialists lean on its ability to disrupt microbial growth where cleanliness counts. The structure packs two methyl groups attached to a benzene ring in the right spots, creating just enough separation in reactivity compared to other xylenols. Labs, hospitals, and factories rely on this compound for its no-nonsense effectiveness. People who have worked in chemical plants know how these ubiquitous phenols become crucial building blocks added into everything from resins and disinfectants to herbicide blends. Its reputation for direct antibacterial action made it a mainstay in labs long before many modern chemical options arrived.
3,5-Xylenol appears as a colorless to pale yellow crystalline solid with a distinct tar-like odor that stays in memory after just one whiff. Its melting point hovers near 64°C, with a boiling point up at about 222°C. It dissolves reasonably well in alcohols, ethers, and most organic solvents, while water solubility comes in low. The methyl groups flanking the phenol ring lower its reactivity slightly compared to plain phenol, but the molecule holds onto classic phenolic acidity. The density sits at around 1.04 g/cm³, which makes solutions of it just heavy enough to demand careful handling in dosage systems. The compound resists light breakdown, retaining stability under normal storage, though inhalation or skin exposure proves hazardous due to phenolic content.
Manufacturers who produce 3,5-xylenol stick to tight technical standards. Chemical purities hover at or above 98 percent for most laboratory-grade batches, with moisture and residual solvent limits spelled out on specification sheets. Suppliers must label each container with CAS numbers, batch IDs, hazard pictograms, and concentration details — no space for confusion. In my years working with chemical supply chains, mislabeling or sub-par documentation always led to headaches: inspectors and safety officers rarely forgive errors when handling phenols. Containers, often made from HDPE or amber glass, protect the compound from UV exposure as well as leaks. Safety Data Sheets (SDS) provide not just the basics but also clear spill and exposure procedures, reflecting the lessons learned from decades of accidents and close calls. It often travels in 25 kg drums, though smaller sealed packs wind up in research labs.
Most often, 3,5-xylenol comes from methylation of phenol using methylating agents like methanol in the presence of aluminum or zinc-based catalysts. Skeptics may question why not just extract it, but natural coal tar routes produce a jumble of isomers needing energy-intensive separations. So, chemical industry labs run tailored alkylation reactions under pressure, followed by fractional distillation to separate 3,5-xylenol from close relatives. These columns demand careful monitoring because over-methylation or catalyst poisoning can tank yields. Careful control over temperature and reactant ratios keeps byproduct formation down. Some greener chemistry approaches focus on tuning catalysts for higher selectivity, as firms eye both cost savings and regulatory demands for lower emissions.
The phenolic hydroxyl group at the heart of 3,5-xylenol opens the door to a range of modifications. Acylation with acid chlorides or anhydrides offers ways to create new esters and ethers better suited for specialty resins or pharmaceuticals. Halogenation with bromine or chlorine introduces further versatility, letting chemists craft tailored fungicides or flame-retardants. Its methyl groups block ortho and para substitution, pushing reactions to ring positions less likely to trigger unwanted crosslinking. I recall more than one project where we tried tweaking reactivity by shifting those methyl groups — small changes in structure brought big swings in performance. In the factory context, 3,5-xylenol acts as a stepping stone for adding bulkier functional groups, ultimately impacting the mechanical or antimicrobial properties of finished products in ways plain phenol can't match.
The chemical nomenclature world brings a long list of alternative tags for 3,5-xylenol. Known in official databases as 3,5-dimethylphenol, it surfaces under trade names like 5-hydroxy-m-xylene, m-xylenol, and meta-xylenol. The CAS number 108-68-9 stays consistent across the board, acting as a non-negotiable key for supply chains and researchers. In Europe, some suppliers might call it m-xylenol or add local language twists. If you’ve spent any time sourcing chemicals across borders, you know names shift, but the molecular formula C8H10O and the ring structure always tell the real story.
Dealing with 3,5-xylenol takes serious attention to safety. Direct contact causes skin and eye burns, with fumes leading to respiratory irritation and, on bad days, central nervous system effects. Training fresh hires in chemical handling means running through protocols for phenols — never skimp on gloves, goggles, or fume hood enclosures. Regulatory agencies enforce workplace exposure limits, usually setting PELs (Permissible Exposure Limits) near 5 ppm. Facilities must keep spill kits, neutralizing agents, and eye wash stations close. Even routine disposal of xylenol waste requires proper incineration or chemical neutralization. Decades of research into workplace incidents pushed companies to lean hard on automation and batch testing, and supervisors rarely tolerate shortcuts during tank transfers or maintenance.
Industries take advantage of 3,5-xylenol’s tough stance against bacteria and fungi. Formulators add it to antiseptic soaps, liquid disinfectants, and even wound cleansers. Municipal water plants use it in smaller doses to keep bacterial films out of pipes and tanks. Agricultural chemical companies renew interest about every five years as regulations shift and crop protection recipes demand a reliable antimicrobial backbone. Material scientists use 3,5-xylenol as a monomer in high-performance resins for automotive interiors — thanks to its resistance to UV and biological breakdown. In factory settings, it winds up in metalworking fluids where bacterial slime can wreck expensive equipment. If you’ve walked a modern production floor, you know these byproducts build up unless someone breaks the cycle — phenolic agents like 3,5-xylenol get that job done.
Laboratories put 3,5-xylenol through tough paces looking for next-generation uses. The search for safer, broad-spectrum disinfectants puts it under the microscope. Researchers also look for ways to reduce environmental and health risks tied to phenols, working on gentler derivatives and improved delivery systems. Polymer chemistry teams use it as a branching point to design faster-curing resins and more durable plastics — often seeking a balance between performance and lower toxicity. Years spent working on collaborative industrial R&D projects taught me the real innovation comes from cross-disciplinary insight. Biologists, process engineers, and chemists exchange findings about byproduct handling, new reaction paths, and potential market expansions. That broad approach leads to smarter, cleaner, and more adaptable chemical products.
Animal studies and occupational health reports point to real concerns with 3,5-xylenol exposure. Inhalation at high levels produces headaches and neurological symptoms, while prolonged skin contact leads to burns and systemic toxicity. Chronic use in poorly ventilated settings compounds risks: case reports document liver and kidney stress after repeated exposures. Regulators base their workplace limits on these findings, forcing industries to re-examine ventilation systems, personal protective equipment, and emergency protocols after each safety review. Environmental toxicologists measure the breakdown products in water and soil, tracking persistence and bioaccumulation. Treating chemical injuries often means rapid decontamination and symptomatic care. Research continues into less harmful analogs and better detection methods for tracking early exposure in workers, always pressing the chemical industry to do better.
The outlook for 3,5-xylenol pulls in two directions. On one hand, rising demand for powerful antimicrobials in healthcare, sanitation, and agriculture keeps its role safe for now. On the other, regulatory scrutiny tightens as new data on toxicity and persistent pollution comes in. The next wave of progress isn’t just about higher-purity batches or bigger capacity; it’s about safer substitutes, waste minimization, and rapid detection of trace contaminants in finished goods. In environmentally focused research labs, chemists look for ways to create biodegradable analogs that deliver the same performance without hazardous byproducts. In all, as the chemical landscape changes, 3,5-xylenol’s standing reflects the push-pull of innovation, regulation, and real-world safety — an evolution echoed by anyone who’s ever had to balance performance with health and environmental responsibility on the job.
3,5-Xylenol shows up in a lot of places most folks don’t expect. My time spent in a chemical plant let me see firsthand how it’s handled. Its main job falls in the realm of disinfectants and antiseptics. This chemical does a number on bacteria and helps clinics and hospitals keep their tools clean. Anyone who’s had a minor procedure or cleaned a wound with a hospital-grade cleaner has likely come in contact with a molecule that contains a variant of xylenol, including this one.
Households lean on cleaning agents, and many of the stronger ones rely on 3,5-Xylenol’s ability to break down stains and kill germs. In factories, workers add it to lubricants and metalworking fluids. The reason: it stops microbes from growing. Machinery runs smoother and parts last longer. I’ve watched engineers trust these chemical blends to keep equipment steady, knowing a breakdown costs more than a good cleaning agent does.
Paint shops use 3,5-Xylenol too. It slows down fungal growth in paint. Once, a friend working as a home renovator pointed out that certain wall primers smell odd for a reason—they use chemicals like this one to help paint last. Boat hulls and fences also stay clean longer because manufacturers use these additives when they mix up paints and coatings.
Drug-resistant germs remain a huge threat in healthcare. The World Health Organization calls out hygiene and infection control as top priorities. In clinics, every wipe-down of a surface reduces the risk of an outbreak. That’s where 3,5-Xylenol delivers value. Hospitals need affordable, proven chemicals, and this one has stood up against many bacteria and fungi strains. Years ago, during a flu outbreak in my city, supplies thinned out. Clinics leaned even more on strong surface disinfectants to keep the virus from spreading between rooms or patients.
Handling 3,5-Xylenol takes care. Spills can harm local waterways. Fish and plants don’t handle phenol chemicals well. At work, I saw strict storage rules in place—locked cabinets, clear labeling, and daily logs. Many local regulations set discharge limits. It also causes skin irritation at high strengths, so gloves and masks are standard. People mixing or spraying it for cleaning get taught to follow the rules every time.
Some companies and cities have pushed for “greener” cleaning solutions. Safer alternatives get built on plant oils or enzyme-based products. Still, issues come up when moving away from what works. Until science offers replacements that tackle both strong germs and cost, 3,5-Xylenol holds its ground. I’ve watched my neighborhood clinic field-test new products. Some made things shine, but didn’t kill all the germs. Teams stick with what gives real protection first—and they demand long-term safety data.
The challenge: balance. Good hygiene saves lives, but so does clean water and safe surroundings. Factories and clinics use chemicals like 3,5-Xylenol because they work well and don’t break the budget. Strict handling, less waste, and smart disposal can lower risks and keep both people and environments healthier. Keeping up with better solutions means listening to new research and using strong tools without ignoring their side effects.
No one wants surprises in the lab or manufacturing line. 3,5-Xylenol, a chemical used in disinfectants, dyes, and even paint, pops up in more places than most expect. Hearing its name may not set off immediate alarm bells, but handling it carelessly can bring real problems. I’ve mixed enough cleaning agents and tested enough samples in the lab to trust both caution and experience over guesswork in situations like these.
Let’s not ignore what’s clear: 3,5-Xylenol can irritate skin, eyes, and the respiratory tract, according to research published in regulatory journals (OSHA, NIOSH, and the International Agency for Research on Cancer). Getting the powder or fumes on your skin or in your lungs brings risks most regular people underestimate, and unfortunately, once you notice symptoms, your body’s already feeling the effects.
More studies highlight that prolonged or repeated contact causes dermatitis, headaches, or breathing trouble. Many seasoned chemists or custodial workers will recall awkward rashes, minor burns, or coughing fits from getting too close to strong phenolic compounds like this one. Safety data sheets—those long packets no one loves—repeat warnings for good reason: chemical burns, severe eye damage, and internal problems happen without the right precautions.
In decades of work, I've noticed people get in trouble mostly by skipping gloves or working quickly instead of methodically. Some folks underestimate what vapors can do in an indoor space, or they don’t bother checking if their eye protection is snug. The people who walk away with health intact are usually those who pay attention to routine. Safety goggles might steam up, but they block that one stray splash. Fume hoods buzz loudly, but they save lungs from the sting of potent vapors.
Ignoring the risks of 3,5-Xylenol is not just about hurting yourself. Businesses eat heavy costs for workplace injuries—lost time, insurance premiums, and fines for violating chemical safety laws. Lives become more complicated, not just with the pain, but with paperwork, retraining, and damaged trust. No one on a team wants to finish the day in the emergency room over something that started as a minor spill or skipped glove.
Effective solutions exist, and I’ve seen them turn dangerous situations around. Use of personal protective equipment—gloves, goggles, lab coats—goes a long way. Ventilation matters just as much. Work in well-aired spaces and avoid storing leftover chemicals in open containers. Training never feels new and shiny, but people who stay updated on instructions and rerun emergency drills make fewer mistakes. Label everything clearly and store 3,5-Xylenol away from food areas or flammable substances.
Routine self-checks matter: before starting a task with this chemical, ask yourself if your safety materials are ready and your plan clear. If any uncertainty remains, stop and consult a supervisor or safety officer. Prioritizing safety reflects not just care for oneself but respect for everyone who shares the space. In handling 3,5-Xylenol, knowledge and routine win every time.
Ask anyone who’s ever worked with cleaning agents, antiseptics, or industrial chemicals about 3,5-Xylenol, and you’ll get more than one cautionary tale. Used in a range of products from disinfectants to chemical intermediates, this compound can create health problems nobody should ignore. If you’ve handled this chemical, you remember the sharp smell, the sting on bare skin, maybe even those bouts of coughing in a poorly ventilated space. That’s no coincidence—3,5-Xylenol carries risks, some more subtle than others.
For starters, this chemical stands out as an irritant. Direct contact with the skin causes redness, swelling, and sometimes blistering. Workers mixing or spraying it often talk about burning sensations, sometimes followed by skin that peels or cracks. It isn’t just a surface-level problem; absorption through the skin can lead to deeper issues, especially with repeated exposure.
Inhalation presents another real risk. Shortness of breath, coughing fits, wheezing—these show up in reports from those using poorly protected equipment. Many industrial hygiene specialists urge respirators or better ventilation for a reason: 3,5-Xylenol vapors cause acute respiratory tract irritation and can make existing asthma much worse. NIOSH and OSHA have posted warnings for years, with studies pointing out inflammation and even pulmonary edema at high exposures.
Some side effects go beyond the obvious. I’ve heard from people who started to feel dizzy, nauseous, and lightheaded after long hours working with concentrated solutions. Toxicologists link this to the chemical’s ability to depress the central nervous system. In severe cases, people describe confusion and loss of coordination—symptoms that mean a hospital trip, not just a coffee break.
Long-term contact isn’t just uncomfortable, it’s dangerous. CDC records highlight repeated exposure as a factor in persistent dermatitis, chronic headaches, and sometimes even kidney and liver strain. The body tries to metabolize chemicals like 3,5-Xylenol, putting those organs under stress over time. There’s ongoing research about the potential for carcinogenic effects, but current evidence lands more on chronic irritation than cancer.
A splash of prevention does more than a gallon of cure. I’ve seen too many workplaces treat gloves and goggles as optional extras, only to regret it later. Proper training changes everything. Engineering controls, protective equipment, and clear labeling cut exposure. Changing habits—the way people transfer and store chemicals—brings down accidental contact. Companies using this chemical should revisit their safety data sheets, hold regular training, and check on ventilation systems.
As for individual responsibility, checking for rashes, respiratory problems, or unexplained fatigue shouldn’t fall down the priority list. Quick access to wash stations, eye rinse bottles, and first aid kits matters on days when things go wrong. Staying proactive—asking about safer alternatives or lower concentrations—gives workers power over their own health.
3,5-Xylenol isn’t unique in its risks, but being careless turns an everyday chemical into a health disaster. Folks handling it deserve frank talk, practical steps, and respect for the reality they face on the job.
A lot of folks in labs and industrial jobs deal with stuff like 3,5-xylenol. It might sound like something only for chemists, but it finds its way into cleaning products, disinfectants, and even certain pesticides. Most of us never think about where these chemicals land between uses, yet the simple act of storage often makes the difference between safe handling and a potential headache.
3,5-xylenol, or m-xylene-5-ol, doesn’t look especially worrisome at first glance – just a pale yellow solid or liquid with a distinctive medical odor. Dig a bit deeper and you’ll notice it can irritate skin, eyes, lungs, and even trigger headaches. Some folks, including myself during lab days, discovered the impact after a poorly sealed bottle left fumes drifting through the workspace. It’s not just about comfort; safety, air quality, and the risk of a fire all come into play if things slip through the cracks.
Direct sunlight and heat play tricks on phenolic compounds like 3,5-xylenol. Leave a container in a warm spot or near a sunlit window, and you push it closer to breaking down or catching fire. In my early career, a colleague learned the hard way after a bottle left on a shelf above a radiator started leaking vapor and set off air monitors. Cool, dry, and well-ventilated spots limit these kinds of surprises. So, cupboards away from heat sources and sunlight—not the back of a cluttered supply closet—work best.
Keepers of these chemicals talk all the time about tight sealing. It’s not an empty warning. Vapors can spread, and contamination can ruin entire batches of sensitive goods. Containers with heavy-duty screw tops or safety seals get the job done. Put them in something non-reactive—glass or HDPE plastic usually handle 3,5-xylenol well. I still remember noticeable staining and warping on a cheap plastic container, which almost led to a shelf cleanup disaster.
Labeling is another step that always sounds boring until you grab the wrong stuff in a rushed moment. Clear chemical names, hazard pictograms, and date received should stand out. The job gets messy fast if someone mixes things up and thinks 3,5-xylenol can share space with oxidizers or acids. These combinations can spark dangerous reactions, sometimes with only a little humidity in the air. Good labels and a bit of old-fashioned separation stop accidents before they start.
People talk about proper storage, but accidents sometimes find a way. Leaks and spills picked up quickly won’t end in injury. Absorbent pads, available gloves, and plenty of ventilation all help. I’ve cleaned up my share of spills, and fast action with the right gear always beats letting fumes hang around. Anyone handling 3,5-xylenol often should have easy access to safety data sheets and know their emergency contacts.
There isn’t magic in chemical storage, just a steady routine and respect for the risks. Enforced rules, reliable storage gear, ventilation, and a crew that watches out for each other keep things safe in labs and warehouses alike. These habits build trust and accountability, which cut down on both mistakes and worries. Chemical safety links knowledge to everyday practice. My own peace of mind always came from knowing coworkers looked out for the details—tight lids, real labels, and shelves clear of clutter. It’s small steps that prevent trouble and let people focus on getting the job done right.
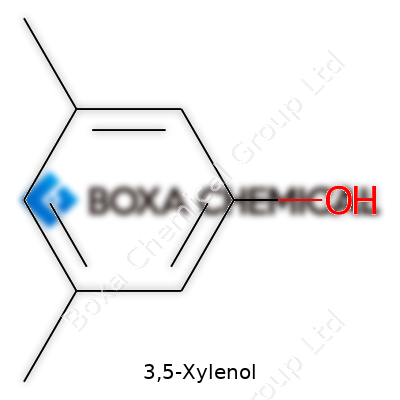
3,5-Xylenol stands out as a simple aromatic compound, one many of us have come across in cleaning agents or antiseptics. Its chemical formula, C8H10O, points to a core structure you’d expect from phenolic compounds. It’s built from a benzene ring holding two methyl groups at the 3 and 5 positions and a single hydroxyl group.
During my lab days, understanding small structural tweaks in organic molecules showed me how physical traits and hazards shift. 3,5-Xylenol’s two methyl groups turn what otherwise would be a plain phenol into something more robust as a disinfectant. The methyl groups increase lipid solubility, which helps the molecule breach bacterial membranes. The hydroxyl part gives it the “phenolic” punch—reacting with cell proteins and causing denaturation.
Factories rely on 3,5-Xylenol as a key ingredient in disinfectants, dyes, and some resins. People at home using germicidal cleaners often benefit from molecules like this, without ever seeing the name on the front label. Hospitals and clinics use phenolic antiseptics to cut down on bacteria in operating rooms.
Growing up, my family ran a small cleaning business. Every week we navigated which products actually made a space smell and feel clean versus which brands simply masked problems. Phenolic compounds like 3,5-Xylenol sat among trusted agents—broad-spectrum, quick-acting, hard on bacteria but relatively forgiving on human skin at lower concentrations.
There’s risk in misusing phenolic disinfectants. Over-concentration can lead to burns or respiratory irritation. Safety guidelines strictly recommend gloves, ventilation, and accurate dilution to avoid harm. Persistent use leaves traces in wastewater; municipal water treatment plants must work harder to break down phenolic chemicals, so run-off matters to the planet.
Research has connected xylenols to some aquatic toxicity. High levels in rivers or streams disrupt fish reproduction. Governments in Europe and North America have set strong disposal rules to keep disinfectants from pooling into local water supplies. Learning about this issue, I started paying closer attention to “green” and “biodegradable” claims on cleaning product labels and always checked for phenolic content when cleaning in tight spaces.
No molecule offers a perfect answer, but innovation grows every year. Biodegradable alternatives and enzyme-based cleaners have started to replace heavy phenolic disinfectants in many settings. Still, hospitals keep molecules like 3,5-Xylenol in their arsenal for dealing with the toughest pathogens. Manufacturers invest in safer ways to produce and dispose of xylenols, curbing environmental impact and exposure.
For public health, balancing strong disinfection power with minimal risk for people and wildlife means continual monitoring and education. Teachers, families, and facility managers choosing these chemicals feel increasingly responsible for understanding what’s behind the ingredient list—a trend worth encouraging for both safety and wellbeing.
| Names | |
| Preferred IUPAC name | 5-methylbenzene-1,3-diol |
| Other names |
5-Methyl-m-xylenol 3,5-Dimethylphenol m-Xylenol |
| Pronunciation | /ˌθriː.faɪv ˈzaɪlɪˌnɒl/ |
| Identifiers | |
| CAS Number | 108-68-9 |
| Beilstein Reference | 604134 |
| ChEBI | CHEBI:17349 |
| ChEMBL | CHEMBL16185 |
| ChemSpider | 11717 |
| DrugBank | DB03620 |
| ECHA InfoCard | ECHA InfoCard: 100.003.120 |
| EC Number | 201-199-9 |
| Gmelin Reference | 7858 |
| KEGG | C01417 |
| MeSH | D015590 |
| PubChem CID | 6997 |
| RTECS number | ZE2450000 |
| UNII | M7XE862UW1 |
| UN number | UN1672 |
| Properties | |
| Chemical formula | C8H10O |
| Molar mass | 122.16 g/mol |
| Appearance | White to off-white crystalline solid |
| Odor | Phenolic |
| Density | 1.03 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.9 |
| Vapor pressure | 0.17 mmHg (25°C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 9.37 |
| Magnetic susceptibility (χ) | -72.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.535 |
| Viscosity | 3.74 mPa·s (20 °C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 120.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –115.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –4010 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D08AE04 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye damage, toxic to aquatic life. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Toxic to aquatic life with long lasting effects. |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 3*2*0 |
| Flash point | 113 °C (235 °F; 386 K) |
| Autoignition temperature | 530°C |
| Explosive limits | Explosive limits: 1.3–7% |
| Lethal dose or concentration | LD50 oral rat 3830 mg/kg |
| LD50 (median dose) | 0.5 g/kg (oral, rat) |
| NIOSH | UR7800000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of 3,5-Xylenol: "5 ppm (20 mg/m3) TWA |
| REL (Recommended) | 730 mg/L (rat, 4 hours) |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Phenol o-Cresol m-Cresol p-Cresol 2,4-Xylenol |