3,4-Xylenol showed up on chemists’ radar over a century ago, in the wave of discoveries swirling around coal tar. Back then, people were fascinated by the mixture of aromatic hydrocarbons coming out of coal processing. Among the variety of phenolic compounds isolated, scientists noticed some stood out for their antiseptic or solvent powers. In those days, this compound was just another drop in a chemical soup, but the right minds saw potential. Through trial and error at the lab bench, the uses for these methyl-substituted phenols started to come into focus, laying the groundwork for what we know about 3,4-xylenol production and handling today.
The core of 3,4-xylenol lies in its function as an industrial intermediate. Factories rely on it for not just one or two specialties but a list—from disinfectants and resins to dyes and agrochemical products. In manufacturing, versatility counts more than name recognition. Over years of hands-on application, industry players learned to value this compound for its robust performance in both specialized technical environments and more standard production lines.
3,4-Xylenol usually appears as colorless to pale yellow crystals when pure. Anyone who deals with phenolic compounds knows the smell: harsh, like tar and hospital aisles combined. It melts around 75°C, boiling at roughly 220°C, and dissolves in organic solvents, but not especially well in water. The methyl groups at positions three and four on the benzene ring make it more lipophilic than simple phenol. This tweak influences not just the handling but also the types of reactions possible and where it finds utility. Despite its strong odor and tendency to stick around if spilled, it keeps a stable profile under normal storage.
Product purity, typical melting point range, water content, and permissible trace contaminants define most technical grade 3,4-xylenol available today. Industrial buyers often look for specification sheets listing at least 99% purity and minimal sulfur or heavy metals content. In the warehouse or transit, the barrels and containers carry hazard pictograms for corrosive materials and toxic substances as per GHS guidelines. Compliance with international transport and workplace standards, including UN codes, gets taken seriously to avoid shipping delays, fines, or worse—accidents that put workers’ health on the line.
Standard synthesis of 3,4-xylenol starts from m-xylene via sulfonation, followed by alkaline fusion. Chemical producers discovered early on that tweaking reaction temperature and pH gives better yields and purer product. Alternative routes tap into nitration and subsequent reduction and hydrolysis, but these methods demand more steps and extra waste management. Efficient catalysis and careful separation remain technical challenges with room for improvement, both from cost and safety angles. In practice, big plants tweak process parameters to cut down energy and waste, learning from previous runs and ongoing research.
Having two methyl groups ortho and para to the hydroxyl makes 3,4-xylenol useful for crafting more complex molecules. It stands up to alkylation, acylation, and etherification. This flexibility feeds applications into textile dyes, UV absorbers, and specialty polymers. Seasoned chemists appreciate that the methyl substitutions shift both reactivity and selectivity in downstream reactions, opening unique routes in aromatic chemistry related to pharmaceuticals and advanced materials. Where others run into bottlenecks with basic phenol, this compound’s branching structure solves problems.
Through the years, 3,4-xylenol traveled under several aliases. In chemical catalogs, you’ll find it listed as 3,4-dimethylphenol, m-xylenol, or even by older trade names. Industry insiders and seasoned suppliers spot these names right away, ensuring product traceability on datasheets and inventories. Mix-ups can happen in purchasing or supply chains, so keeping an eye on these labels can mean the difference between a clean production run and costly downtime.
Every worksite handling this chemical follows strict protocols. Skin and eye contact risks serious irritation. Inhalation carries risks for respiratory effects, sometimes leading to more severe symptoms if controls lag. Regulations require proper ventilation, gloves, goggles, and in some cases, full respirators. Storage regulations set minimum distance from strong oxidizers, ignition sources, and food storage. Waste streams undergo detailed hazard identification before disposal, as environmental authorities keep tight rein on phenolic pollutants. Worker training remains critical; regular drills help prevent routine slips from becoming dangerous incidents.
Disinfectant and antiseptic production remains a main draw for 3,4-xylenol, especially in countries balancing cost and robust microbial control. Paints, resins, and adhesives reach higher performance with this compound added for extra crosslinking or antibacterial effects. It features in the formulation of agricultural fungicides and veterinary drugs, where its stability and action offset other ingredients’ weaknesses. Specialty chemical companies feed it into synthesis routes for active pharmaceutical compounds and flavor intermediates, wringing maximum value from its unique structure. No matter the sector, reliability and proven record keep it on buyers’ order sheets.
R&D circles keep looking for cleaner syntheses, lower-toxicity derivatives, and formulation tweaks that cut costs and environmental impact. Universities and industrial labs run comparative studies on new catalysts and green solvents, chipping away at hazardous byproducts and reducing overall reaction times. Integration with digital tools—machine learning and high-throughput screening—has already identified promising new applications, especially for surface coatings and medical device sterilization. Collaborations between academic teams and specialist manufacturers often set the pace, translating advances into scaled-up protocols ready for industry pilots.
Ongoing animal studies and workplace exposure tracking show phenolic compounds in this class, including 3,4-xylenol, pose both acute and chronic risks. Oral, dermal, and inhalation exposure may lead to tissue irritation and, at higher levels, deeper organ impact. Years of occupational health research support strict exposure limits in most jurisdictions. Carcinogenicity studies remain inconclusive, but the potential for mutagenic effects means technical staff insist on up-to-date material safety data and ongoing monitoring. Toxicology experts lean on laboratory and field data, updating best practices whenever new findings emerge.
Sustainable production wins attention in industry meetings and policy talks. Technical teams work to shrink the environmental footprint of synthesis while regulators tighten rules around phenolic waste and emissions. Demand from medical, agri-chemical, and materials science stays solid, encouraging broader investment in new process technology. Some researchers see potential for engineered biocatalysts to make synthesis cleaner and cheaper. As digitalization spreads through laboratories and plants, faster discovery and safer handling methods look much more achievable than before. Navigating these shifts puts chemical engineers and research chemists at the center of finding practical answers, shaping the next chapter for 3,4-xylenol and its broader class.
3,4-Xylenol, also called 3,4-dimethylphenol, stands as a chemical compound often spotted in discussions about disinfectants and chemical synthesis. The name might not ring a bell for most folks, but it finds its way into daily life more often than most realize. The compound appears as a colorless to pale yellow crystalline solid, with a sharp, characteristic phenolic smell that’s hard to miss if you’ve ever encountered laboratory-made cleaners.
You might not see 3,4-xylenol on the front label at the supermarket, but this compound plays a solid role in disinfectants, especially in products used by hospitals and janitorial crews. Where I’ve worked as a janitor in a busy building, I’ve seen cleaning products turn up with cresols and xylenols among their ingredients. These phenolic compounds go after bacteria and some viruses fast. Studies show they break down cell walls, so they help cut down germ spread in tough environments — think shared toilets, locker rooms, and clinics. This isn’t about flashy marketing. The effectiveness remains proven, with research by the World Health Organization listing xylenols as active ingredients for surface disinfection during outbreaks.
Beyond cleaning, 3,4-xylenol matters to the folks who make resins, plastics, and other chemicals. I had summer work in a plant that cranked out phenolic resins, and xylenols were flown in on tankers. As one of the main raw materials, the compound kick-starts reactions needed for adhesives, coating materials, and the electronics world. Phenol-based resins wouldn’t match their present strength or flexibility without this “building block.” Automotive coatings, chip boards, and electronics assemblies all make use of these advanced resins. If you’ve ever held a circuit board, there’s a good chance the initial steps included chemicals like 3,4-xylenol.
Of course, not everything about 3,4-xylenol works in our favor. Handling this stuff in the lab or on the shop floor means putting on protective gloves and goggles. Splashing it on skin or breathing the vapors can irritate your skin, lungs, and eyes. Chronic exposure leads to more serious health issues, including organ damage. The National Institute for Occupational Safety and Health warns about the danger of frequent contact, so personal protective gear and good ventilation matter. It’s not something to take lightly — and from past experience, I’ve seen co-workers develop rashes after short exposure without gloves.
3,4-Xylenol does not break down easily in water, so it can collect in soil and groundwater. If a facility dumps waste without treatment, waterways near industrial sites may end up with toxic residue. Fish and other aquatic life suffer damage or die off, leading to bigger environmental headaches for the community. Environmental agencies like the EPA set limits on how much can be present in waste streams, but enforcement gaps still happen far too often. I grew up near an industrial site with a faint chemical smell in the creek — and runoff remains a serious concern for families nearby.
Growth in green chemistry points toward safer antimicrobial agents. Companies keep searching for cleaning solutions with less harsh side effects for workers. At the same time, plants now install scrubbers and better waste treatment systems. Strong training for handling hazardous substances and regular health checks go far in protecting workers. Choosing cleaners that use alternative, biodegradable agents also helps slow the build-up of persistent chemicals in the environment. Everyone — workers, consumers, and regulators — holds a role in limiting the risks and keeping both people and the earth safe.
Chemicals drift through daily routines more than people notice, and 3,4-xylenol pops up in many settings. Labs use it, factories rely on it, cleaning agents carry its traces. This common presence can shape people’s thinking: if it shows up so much, maybe it isn’t harmful. The facts don’t line up so neatly, though.
3,4-Xylenol, also called methylphenol, belongs to the phenol family. Many phenols bring some danger—they irritate skin, eyes, and breathing passages. The science backs this up. Research by the National Institute for Occupational Safety and Health shows that 3,4-xylenol, even in small doses, can cause redness, burning, or corrosion when splashed on skin. Breathing in the fumes causes headaches and stings the nose or throat.
In the workplace, technicians who handle this chemical without gloves or proper ventilation soon feel the burn. It doesn’t take a spill for symptoms to start. A wiped counter, or a container opened in a poorly ventilated space, brings risk. Workers talk about quick symptoms: burning sensations, nausea, dizziness. These are not rare.
Eye exposure especially causes trouble. Chemicals like 3,4-xylenol can corrode the outer eye. In my own years working in a lab, a single drop from a glass pipette left a mild sting that needed flushing for several minutes. Even prompt rinsing sometimes leaves lingering discomfort. Cases of severe exposure report blurred vision, tissue damage, and a need for urgent medical care.
Long-term health effects bring more uncertainty. Animal studies report liver and kidney problems after repeated exposure. No clear proof exists on its cancer-causing power in people, but chronic inflammation and chemical burns often make long-term work in certain jobs hazardous. The U.S. Environmental Protection Agency and European Chemicals Agency both list 3,4-xylenol as hazardous, requiring warning labels and careful handling.
The potential for genetic damage has worried scientists, too. Animal tests found some signs of DNA damage at high concentrations, though typical use levels in industrial settings sit below these. Still, many companies adopt strict guidelines and health checks to spot early warning signs among workers.
Accidents and exposure often come down to missing basics—windows that stay shut, gloves that tear, skip-the-mask shortcuts. Better ventilation and strict use of gloves, masks, and splash shields help control exposure. In some modern facilities, chemical fume hoods have become standard. Employees trained in emergency rinsing techniques, such as using eyewashes or showers near workspaces, face less risk of severe injury.
Simple changes at home or in schools matter, too. When products containing 3,4-xylenol leave their industrial birthplace and appear under the kitchen sink or in art projects, a quick safety check can save pain. Reading the label, airing out a room, and using gloves create layers of protection that never seem worth skipping—until the burn starts.
Manufacturers have started to push alternative chemicals with fewer side effects, especially in household cleaning. Regulations nudge companies toward less hazardous compounds. These steps come from people reporting their health problems, from regulators tracking evidence, and from a growing understanding that even familiar chemicals deserve careful respect. Every chemical brings both benefit and risk, and the real job lies in keeping the scales from tipping the wrong way.
3,4-Xylenol isn’t something to store alongside everyday supplies. Workers know its sharp odor and irritating vapor. Even a brief encounter leaves eyes watering and brings coughing. This chemical shows up in labs and, sometimes, as a cleaner or intermediate. The moment a drum or bottle arrives, everyone’s safety practices deserve a look. Consistently following those guidelines has helped me avoid close calls in both small workshops and industry-scale storerooms.
I prefer a cool, dry storeroom with solid ventilation. Moisture invites oxidation and, left unchecked, could change the properties or even produce dangerous byproducts. Warm rooms turn slight leaks into clouds of vapor. If you’re stacking chemicals, keep 3,4-Xylenol away from acids, oxidizers, and combustibles. Mixing with peroxides, say, can spark reactions you definitely want to avoid.
Metal shelves with a corrosion-proof coating make a huge difference. I’ve seen what leaks and spills do to plain steel or wood. Staining is the least of the problems—wood soaks up liquid, spreads fumes, and even turns into fuel in a fire. If you add spill trays under every container, you’ll save time on cleanup and avoid slip hazards. Never stash chemicals above eye level. Anything that spills overhead can end up on your face or body in seconds.
Any bottle without a label goes straight to disposal in my book. Record every date, concentration, and hazard warning right on the bottle. In one shop, we used big, bold letters and waterproof tape. Regulators want labels, but so do your coworkers—mistakes happen fast when things get busy. Lock cabinets and restrict access. Folks with little chemical training are often the most at risk for accidents. If in doubt, only let trained handlers grab those bottles.
Gloves, goggles, and a thick apron are not optional. I know people who skipped PPE for “just a minute” and wound up with burns and breathing trouble. Airflow matters. Without it, fumes hang around and pile up—especially in basements. Add a fume hood for pouring and mixing. Near storage, keep baking soda handy for small spills and a full-scale chemical spill kit for anything bigger. If you see a leak, alert the team and follow the kit’s exact directions.
We ran regular drills. Every shop, lab, and warehouse should do the same. Familiarity with emergency eyewash stations saves lives. I once saw a person freeze in a panic—practicing for emergencies helped them act faster next time.
Waste never goes in the drain, trash, or recycling bin. Hazardous waste collection days aren’t just a nuisance—they keep the wider community safe. In my experience, even small chemical residues cause trouble if someone tries to burn or landfill them.
Clean storage spaces limit exposure and stop old drips from becoming ongoing hazards. Routine checks for cracks, corrosion, and signs of leaks pick up problems early. A little effort up front saves time, money, and sometimes, people’s health.
Laws around chemical safety exist for good reasons. Respecting them protects workers and nearby neighborhoods, not just the company’s bottom line. The best workplaces I’ve seen put real effort into training and regular oversight. That’s what keeps everyone safe.
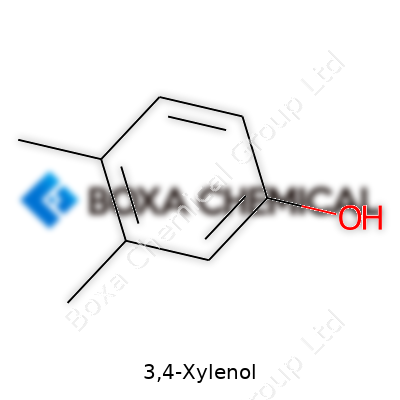
3,4-Xylenol's chemical formula is C8H10O. It often pops up in chemistry labs and can be found in textbooks when reading about phenols. This particular isomer takes its name from the two methyl groups sitting on the third and fourth positions of the benzene ring, right next to a hydroxyl group. Anyone familiar with phenol chemistry might appreciate just how those positions affect its reactivity. The structural formula spells all this out: a benzene ring with two methyl (CH3) groups and a single hydroxyl (–OH) group. Skipping straight to the point, C8H10O means it has eight carbons, ten hydrogens, and a lone oxygen.
3,4-Xylenol shows up as colorless or pale yellow, crystalline solid—it doesn’t look like the more iconic white powder people expect from many lab chemicals. Sometimes it takes the form of flakes, especially if it’s been sitting around for a while. Over time, exposure to air or light may deepen its shade to a faint yellow, but fresh samples almost always appear nearly colorless. The crystals break apart pretty easily—this matters for anyone handling it with gloves in the lab, since they’ll feel almost soap-like, slightly greasy.
One sharp detail: it gives off an odor that’s phenolic and medicinal, not unlike carbolic soap. If you’ve worked with disinfectants or some old-school antiseptic products, you’ll recognize the smell straight away. This comes from the phenol backbone—it’s tough to mistake once you’ve had the experience.
The utility of 3,4-xylenol doesn’t come from appearance alone. Take the traditional uses in disinfectants and cleaners. It played a big role before modern substitutes arrived, and still finds a spot in specialty applications. Chemists and engineers don’t choose it for its looks—they value its strong antibacterial properties. People have focused plenty on phenol-based compounds to control bacteria and fungi in both industrial and consumer settings. As new strains of bacteria pop up resistant to common agents, returning to “older” compounds like 3,4-xylenol sometimes feels necessary.
Workers in production facilities must pay attention to the solid’s appearance and purity—impurities can change melting point or performance. Analytical chemists use simple appearance checks alongside gas chromatography or spectroscopic analysis. A slight change in color, or an unexpected texture, often hints at contamination or decomposition. Lab skills honed from years of handling such chemicals make a big difference, and experience matters just as much as technical guides.
Handling compounds like 3,4-xylenol in the workplace or research calls for strong protocols. Direct skin contact brings risks: the irritation isn’t just theoretical, so gloves and proper ventilation aren’t optional. Most facilities these days lean on established procedures and updated safety data to keep people safe. Engineers can invest in closed systems or better storage to prevent exposure or unwanted reactions. For outreach, clear and direct training helps prevent careless mistakes—even a seasoned chemist skips shortcuts when dealing with strong phenols.
People learning about chemicals for the first time sometimes brush off appearance, thinking formula or data sheets tell the full story. My experience shows otherwise. Small details like color, feel, and especially odor add up, shaping decision-making from the classroom to high-tech manufacturing. That’s why real-world observation and hands-on knowledge of compounds like 3,4-xylenol deserve respect alongside the numbers and chemical equations.
Plenty of chemicals look harmless, yet they hide a mix of risks. 3,4-Xylenol belongs to that club. This compound shows up a lot in labs and some industrial settings. Touching or breathing in its fumes can sting your eyes, burn your skin, or start headaches. You splash it on the ground, you risk cancer for tomorrow and polluted water or poisoned soil almost instantly. Each drop that runs down a drain punches through municipal treatment like it was tissue paper. It doesn’t just disappear; it tags along into environments where it messes with fish, ruins microbes, and pushes health systems to pick up the slack.
Stacks of leftover bottles sit in old stockrooms everywhere. People might think about pouring it down a sink or tossing containers in trash bins. That sort of shortcut becomes dangerous almost right away. Once down the pipes, the stuff lingers in waterways. It travels from city sewers into rivers, out to wherever local water flows. Wild creatures pick it up, and it sometimes sneaks into tap water. Direct trash disposal isn’t safer. Trash trucks can leak, asphalt can break, and soon toxic puddles pop up in parking lots. Dumpsters aren’t built for chemicals—they don’t contain, decompose, or neutralize them.
I remember a chemist once explaining that the right answer almost always costs more, takes longer, and demands more paperwork. That goes double for anything flammable and toxic, which sums up 3,4-Xylenol. Chemical waste handlers know how to store, move, and destroy fluids like this without hurting people or the earth. Local governments register these handlers, and safety inspectors keep checking their methods. Many companies have to file records about how much of this stuff passes in and out. You contact your local hazardous waste disposal service, explain the material you’re handing over, and let them guide the pickup and transport.
Nobody gets instant pick-up service; sometimes those old bottles wait for months. Keeping chemicals safe during storage helps avoid leaks and fumes. Store them in original containers with tight-fitting lids. Avoid metal shelves, which can corrode. Lock them in a chemical cabinet, away from sunlight and heat. Even minor spills can turn into big headaches, so plug up any container cracks and slap on extra hazard labels. Room ventilation means staff don’t choke on vapors if something slips open by mistake.
Most problems start with people skipping steps because they never learned the risks. Good training makes a difference. Workers need reminders on where to store waste, how to deal with spills, and when to tag old stock for pickup. Supervisors shouldn’t just trust labels—they need to check containers and the places they’re kept. A simple walkthrough every month catches leaks, bad seals, and forgotten stock. Prevention beats panic every time.
The story isn’t only about safe cleanup. Using less toxic material cuts down on accidents in the first place. Some labs swap 3,4-Xylenol out for greener substitutes or recycle it with specialized equipment. This keeps disposal costs down and reduces exposure across the board. People think about waste at the end, but the best win comes early: skip the risk, and there’s less to worry about.
| Names | |
| Preferred IUPAC name | 3,4-dimethylphenol |
| Other names |
3,4-Dimethylphenol 1-Hydroxy-3,4-dimethylbenzene |
| Pronunciation | /ˈθriː.foʊr.zaɪˈliːn.ɒl/ |
| Identifiers | |
| CAS Number | 95-65-8 |
| Beilstein Reference | Beilstein Reference: 1209223 |
| ChEBI | CHEBI:27273 |
| ChEMBL | CHEMBL14201 |
| ChemSpider | 7475 |
| DrugBank | DB14015 |
| ECHA InfoCard | ECHA InfoCard 100.003.195 |
| EC Number | 201-199-9 |
| Gmelin Reference | 8417 |
| KEGG | C01415 |
| MeSH | D022211 |
| PubChem CID | 8695 |
| RTECS number | ZE2450000 |
| UNII | UQN9OCS58K |
| UN number | UN2430 |
| Properties | |
| Chemical formula | C8H10O |
| Molar mass | 122.16 g/mol |
| Appearance | Colorless to pale yellow liquid with phenolic odor |
| Odor | sweet phenolic |
| Density | 1.02 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 2.3 |
| Vapor pressure | 0.37 mmHg (at 25 °C) |
| Acidity (pKa) | 10.15 |
| Basicity (pKb) | 9.99 |
| Magnetic susceptibility (χ) | -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.531 |
| Viscosity | 3.8 mPa·s (25 °C) |
| Dipole moment | 1.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 165.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -19.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4066.0 kJ/mol |
| Pharmacology | |
| ATC code | D08AE03 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H318, H332 |
| Precautionary statements | P261, P280, P305+P351+P338, P309+P311 |
| NFPA 704 (fire diamond) | 3,4-Xylenol NFPA 704: 2-3-0 |
| Flash point | 86°C |
| Autoignition temperature | 530 °C |
| Explosive limits | 1.3–7% |
| Lethal dose or concentration | LD50 oral rat 1210 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 795 mg/kg |
| NIOSH | ZE2625000 |
| PEL (Permissible) | PEL: 5 ppm (Skin) |
| REL (Recommended) | 0.5 |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Phenol 2,4-Xylenol 2,3-Xylenol 2,5-Xylenol 2,6-Xylenol 3,5-Xylenol |