Chemistry has always been a field driven by curiosity and practical needs. 2-Tert-Butyl-1,4-Benzoquinone, a derivative of benzoquinone, entered the scene through the search for specialized oxidizing agents and selective reaction mediators. Back in the days when the expansion of organic synthesis opened up new doors, inventive researchers aimed for molecules that went beyond the basic quinones. Tert-butylation of benzoquinone wasn’t an accident or a mere extension of textbook reactions—it answered the call for a compound with more stability and unique reactivity compared to its less bulky cousins. As labs grew more sophisticated, and new drugs and chemicals began to rely on the subtle differences a tert-butyl group offered, this quinone gained traction not only among academic researchers but also across various chemical industries.
2-Tert-Butyl-1,4-Benzoquinone stands out as a yellowish crystalline solid. It's used by chemists who want selectivity and prefer a reagent that can undergo redox cycling without falling apart too quickly. The tert-butyl group on the ring blocks certain positions, so unlike regular benzoquinone, it doesn’t react with everything in the flask. Its value shines in both routine oxidation steps in organic labs and as a thrust point for more complex syntheses. My colleagues often appreciated its sharper handling—less volatility, less smell, and a longer shelf life mean fewer headaches during storage or use.
This compound displays a melting point reliably measured around 89–93°C, which makes purification through recrystallization straightforward for anyone with basic lab skills. The yellow color serves as a clear signal of its quinone character. Unlike aromatic hydrocarbons that lean toward hydrophobicity, 2-tert-butyl-1,4-benzoquinone counts as only sparingly soluble in water but dissolves well in ether, chloroform, and alcohols. Its vapor won’t choke the air like some smaller quinones, but heating should stay controlled to avoid breakdown. Chemistry-wise, it's got a bit of a split personality: it holds onto its quinone electronic structure, yet the bulky tert-butyl group at the 2-position blocks out a lot of unwanted side reactions and makes its reduction or nucleophilic attack less chaotic.
Suppliers who offer 2-tert-butyl-1,4-benzoquinone typically guarantee purity over 97%, with HPLC or GC analysis verifying this claim. Containers tend to be amber glass or HDPE screw-cap bottles, since exposure to air and light gradually eats away at quality, and this isn’t a compound you want breaking down on the shelf. Proper labeling reflects the chemical's reactivity: “Strong oxidizer,” “Handle with gloves,” and hazard pictograms for irritants remain standard. Lot numbers, expiry dates, and manufacturing sites also appear, as regulations demand clear trailbacks. Such meticulous labeling helps chemists maintain safe and accurate operations, reducing costly mix-ups in both academic research and industrial settings.
Laboratory synthesis of 2-tert-butyl-1,4-benzoquinone usually starts with hydroquinone or p-tert-butylphenol. Researchers prefer Friedel–Crafts alkylation or oxidative routes, using tert-butyl alcohol and catalysts like aluminum chloride. Once tert-butylation sets in, further oxidation, typically with agents such as ferric chloride or silver oxide, turns the starting material into the desired quinone. I've handled reactions where precise temperature control and slow addition of oxidant made the difference between clean yields and gunky mixtures. Purification comes down to crystallization in non-polar solvents, followed by drying under reduced pressure, ensuring the end-product keeps its defined, crystalline identity.
This quinone comes into play as an oxidizing agent and a building block. Chemists leverage its reactivity in Diels–Alder cycloadditions, where it accepts electrons and forges connections that plain benzoquinone cannot, thanks to the side group’s influence. Reduction to the corresponding hydroquinone often kicks off other synthetic schemes, and the tert-butyl group’s presence can steer attacks from nucleophiles, favoring positions otherwise susceptible in the parent benzoquinone ring. Modifying the molecule with other substituents or transforming it into more advanced intermediates allows for a range of derivative compounds—handy when designing custom molecules with finely tuned properties.
In catalogs and literature, you’ll run across “2-tert-Butyl-p-benzoquinone,” and “2-(1,1-dimethylethyl)-1,4-benzoquinone.” CAS numbers often show up (like 98-29-3), which save time searching inventories or databases. “TBBQ” sometimes appears as a shorthand among those who use the molecule regularly, especially in synthetic organic circles. These labels aren’t just bureaucracy; they steer clear of mix-ups and align research findings globally. Once, a simple mislabeling led to a missed deadline in a partner’s lab, making clear that consistent naming systems are more than just a formality.
It’s not an everyday chemical for casual handling. Given its oxidizing capacity, direct contact can cause skin and respiratory irritation, with evidence from workplace case studies highlighting the need for gloves, lab coats, and eye protection. Fume hoods remain essential, not just out of habit—but out of a clear risk that vapors or accidental spills could make a day in the lab memorable for all the wrong reasons. Spills call for swift containment with inert material, and since environmental contamination isn’t trivial, cleanup uses proper waste channels rather than the drain. Safety data sheets advise storage away from strong acids, reducing agents, and ignition sources. These aren’t excessive rules; they reflect real hazards that show up in industrial accident reports.
Researchers in organic synthesis lean on 2-tert-butyl-1,4-benzoquinone for selective oxidations and as a partner in electron-transfer studies. Pharmaceutical chemists include it in the arsenal when stepping toward complex molecular targets, since the tert-butyl group blocks unwanted reactivity and controls selectivity down to a pinpoint. Industrial processes sometimes line it up as a redox mediator or stabilizer, and certain battery development efforts consider its redox cycling properties. It pops up in research on polymers as a precursor and modifies dye production, laser materials, and specialty coatings where quinonoid structures add value. These uses aren’t random—they’re built from practical observations, trial runs, and published results over decades.
Current work looks deeply at the electronic properties of tert-butylated quinones. With better computational tools, chemists predict and model its behavior in redox cascades and asymmetric catalysis. Some teams investigate its role as an intermediate in developing new antibiotics or redox medicine, intrigued by its ability to accept and shuttle electrons in a controlled manner. Patents filed in recent years document efforts to remake portions of the molecule for use in advanced material science, especially for organic electronics and optoelectronic devices. My contacts in R&D note that cross-disciplinary teams, mixing electrochemists and medicinal chemists, see real promise in modifying the base skeleton for new applications.
Toxicology testing points out that 2-tert-butyl-1,4-benzoquinone, like most quinones, reacts with cellular thiols and can produce oxidative stress in biological systems. In cell models, researchers report cytotoxic effects above certain concentration thresholds, prompting attention to careful dosage and waste management. The compound’s interaction with glutathione and other antioxidants raises big questions for its use in any biomedical context. We’ve seen regulators in certain countries request more specific exposure data before any scale-up for pharmaceuticals, leading to more careful in vivo testing and benchmarks for occupational limits. Safety studies continue, with a trend toward detailed metabolic and excretion profiling rather than just short-term cell assays.
Next steps for 2-tert-butyl-1,4-benzoquinone focus on both safety and expanded utility. Green chemistry advocates keep searching for routes that minimize by-products and rely on safer solvents, eyeing both cost and public health. Battery researchers explore its redox properties for stable, high-capacity energy storage—an effort that could someday push this compound from a niche lab reagent to a backbone of renewable power technology. Pharmaceutical teams watch for ways to direct its reactivity at specific enzymes, hoping for breakthroughs in targeted therapies. As climate and sustainability pressures grow, practical, scalable syntheses that cut emissions and waste push forward. These efforts echo a simple truth I’ve seen in labs and plants alike: innovation depends as much on how we handle known compounds as on dreamt-up new molecules. The story of 2-tert-butyl-1,4-benzoquinone is still being written, shaped every day by people balancing tradition with progress.
If you dive even a little into organic chemistry, you run into a lot of compounds with long, technical names. 2-Tert-Butyl-1,4-Benzoquinone stands out among quinones because chemists, scientists, and industry specialists keep bringing it up in their work—especially those who deal with fine chemicals, specialty catalysts, and synthetic organic research. The presence of a tert-butyl group at the second position brings this molecule some extra punch. I’ve come across it plenty while working alongside teams looking for better oxidative reagents and in research labs chasing more selective catalysts for advanced reactions.
In my experience, some people get excited about benzoquinones because they’re good at accepting electrons. That knack means scientists often reach for 2-Tert-Butyl-1,4-Benzoquinone while developing new methods for oxidizing reactions, or launching redox chemistry efforts that need finely tuned reagents. Even major breakthroughs in organic synthesis—like the development of selective oxidation protocols—regularly feature a benzoquinone derivative as a supporting player. This one, with its bulky tert-butyl group, shows up in places where chemoselectivity and mild conditions make all the difference.
On top of that, this compound pops up in the design of new catalysts, especially where controlling electron flow means the difference between a research success and a reaction failure. A lot of organocatalysis work during the past decade has used benzoquinones to shuttle electron density, offering smoother, more controlled outcomes. I’ve learned that researchers in academia and pharma look for molecules like this while they shape up drug candidates or chase low-waste, green chemistry solutions.
It’s not all research papers and theoretical chemistry. This benzoquinone derivative finds its way into the production of dyes, polymers, and even a few specialty batteries. Electrolyte development teams sometimes try it out for its redox behavior, looking to push the efficiency and safety envelope for next-generation devices. In polymer science, certain benzoquinones act as starting points for highly stable, uniquely colored materials. My time collaborating with materials scientists taught me that these compounds bring a whole toolkit of color, stability, and reactivity that pure hydrocarbons can’t manage.
Biological researchers sometimes explore these quinones in studies of enzyme mimicry or as model compounds for natural redox cofactors, though 2-tert-butyl-1,4-benzoquinone usually appeals more where chemical stability tops the list of priorities.
Every time a lab or company adds a synthetic reagent, safety and environmental impact jump quickly into the conversation. Benzoquinones, including this one, demand careful handling. Their strong oxidizing power calls for goggles, gloves, and good ventilation. My own close calls in the lab taught me not to underestimate how fast these reagents will react, not only with the intended molecules but sometimes unexpectedly with plasticware or even solvents.
Sustainable chemistry brings some challenges. While benzoquinones play crucial roles, they sometimes require rare starting materials or leave behind problem waste. More manufacturers now seek recovery and recycling routes—and push for milder, safer protocols to limit byproducts. This push comes from both regulatory requirements and genuine industry concern for long-term health and safety.
The path forward doesn’t mean ditching benzoquinones; it means making their production and use as safe and clean as possible. Investment in green synthesis, better containment, and careful reaction design can help labs and companies unlock benefits without the environmental and health costs. My own view is that curiosity and practical caution walk hand-in-hand: by sharing lessons learned and building cross-disciplinary teams, science gets not just smarter results, but safer and more sustainable ones.
Organic chemistry might seem like a maze, but everything starts making sense with a bit of patience. Take 2-tert-butyl-1,4-benzoquinone. Its name packs quite a bit of information, and anyone who spent hours staring at skeletal formulas in the lab knows that these long chemical names actually serve as a precise address for each atom.
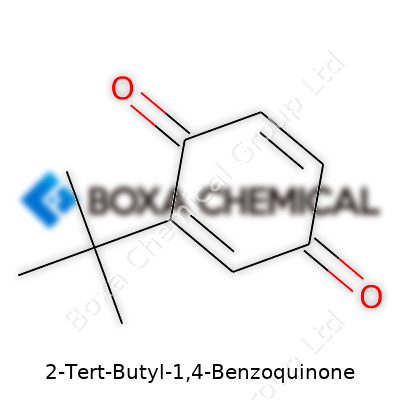
At its core, 2-tert-butyl-1,4-benzoquinone is a derivative of benzoquinone. This molecule relies on the classic six-membered aromatic ring, the benzene ring, which has always been a favorite for chemists and pharmacists alike. But this isn’t your typical benzene. Two oxygen atoms double-bonded to the ring at positions 1 and 4 transform it into a quinone.
The tert-butyl group introduces some character. It’s a bulky, branched chain made of three methyl groups attached to a central carbon atom, and that carbon connects directly to the benzene ring at the ring's second position. In chemistry class, I always found it easier to remember these groups as a sort of shield, sticking out from the carbon framework, pushing neighbors away and sometimes changing how the molecule reacts.
Structure changes everything. The tert-butyl group isn’t just there for show. It bulks up one side of the molecule and, in doing so, it modifies not only its chemical reactivity but also its application potential. For chemists, small shifts like these make molecules easier to track and study in reactions. Even high school students might recognize how adding a bulky group like tert-butyl can shift the entire focus of a reaction—this is the kind of trick that helps drive innovation in creating new materials or medicines.
There’s a reason many pharmaceutical and material science companies pay careful attention to these modifications. Quinones serve as building blocks for dyes, medications, and even in rechargeable batteries. Stick a tert-butyl group on there, and now the stability, solubility, and even biological interactions change. That’s not marketing talk—that’s what research keeps showing, with studies published in sources like the Journal of Organic Chemistry highlighting these property shifts over and over.
With great molecular features come new headaches. The tert-butyl group can make synthesis tricky. It clogs up the reaction site, which often means yield takes a hit. Anyone who’s tried synthesizing substituted benzoquinones knows purification calls for patience. Contaminants sometimes sneak in, and reactions don’t always stick to the easy route the textbooks promise. Even spectroscopic analysis can get muddled as these bulky groups mess with the typical signals.
This is where modern chemistry steps up. Green chemistry initiatives seek to reduce hazardous solvents and streamline purification. Automation in synthesis helps minimize trial and error, cutting down on wasted material. Scientists explore catalysts that help guide the reaction straight to the finished compound without as many side products. These steps save time, but also money—and crucially, result in less environmental impact. Open-access databases let young researchers look up detailed spectra and try new synthesis routes without starting from scratch, a detail I could only dream about a decade ago.
Names in chemistry mean more than just squiggles on a board. In the lab, understanding structure gives clear direction. Tweaking side groups opens doors to better pharmaceuticals, cleaner processes, and advanced batteries. Every branch, like the tert-butyl in this compound, tells a story of trial, error, and discovery—right down to the flask on the bench and the blip on a chromatogram. Knowledge of structures like 2-tert-butyl-1,4-benzoquinone pushes science forward, one bond at a time.
2-Tert-Butyl-1,4-Benzoquinone isn’t a mouthful just for the sake of science. This chemical packs a serious punch, especially if handled lightly. People working in labs or even small-scale production facilities often underestimate the risks of chemicals that aren’t headline-makers. It’s easy to picture a big drum of acid when thinking about danger, but a small bottle of this compound in your glovebox draws just as much concern.
Exposure to this quinone means risking eyes, skin, and lungs. Vapors may irritate airways, and skin contact may burn or stain. For those with a chemistry background, this evokes memories of working with odd-smelling yellow powders and staining hands despite two sets of gloves. Stuff like this will humble anyone who takes shortcuts with personal protective equipment.
Nobody gets extra credit for skipping gloves, goggles, or lab coats. In my own work, nitrile gloves never felt like overkill with chemicals like 2-Tert-Butyl-1,4-Benzoquinone. Safety glasses with side shields, long sleeves, and a proper chemical-resistant apron kept me out of the campus clinic more than once. Don’t trust only latex gloves—they can degrade quickly. Ventilated hoods earn their keep here too; this chemical shouldn’t be hanging in the air where anyone can breathe it.
The tendency with unfamiliar chemicals often leans toward casual shelf placement. This isn’t wise. Avoid sunlight. Oxidation and decomposition accelerate with heat or UV light. Avoid cramming the benzoquinone next to strong acids, bases, or reducing agents. Sturdy, well-labeled bottles kept in a cool, dry cabinet—preferably with a vent or dedicated chemical storage—is basic protocol that prevents worse headaches later.
Chemical spills often trigger a scramble, especially under pressure. Spills with this quinone need more than paper towels and hope. My lab days taught that neutralizing powders and specialized absorbents save time. Scoop up solids with non-sparking tools, as static or friction can be trouble. Cleaning with lots of water only spreads the hazard; stick to proper absorbents. Dispose according to local regulations and treat residues as hazardous waste, not regular lab trash.
Even today, training still lags behind real lab requirements. Materials safety data sheets say a lot, but nothing beats shadowing someone who’s actually handled these compounds. Underestimating a chemical’s effect isn’t just about bravado. Many incidents happen to careful people who simply didn’t get the right instructions—or the right safety gear. It’s not about fearmongering. Sharing what works (and what burns) builds knowledge and accountability.
Science moves quickly, but lessons about safe handling stick around. The facts tell their own story: skin and eye contact mean health risks, fumes can cause harm, and improper waste disposal threatens more than the person dumping it. Institutions like OSHA set occupational exposure limits for similar quinones, for good reason. Each lab or facility must keep safety discussions open, adapt protocols as new info comes in, and make sure new team members learn from those who’ve faced the chemical up close. Investing in protection, training, and safe storage beats any regret.
Stepping into a chemical supplier’s catalog, it’s easy to get lost among technical language. Anyone working in laboratory settings or industrial chemistry knows that purity grades influence every outcome. For 2-tert-Butyl-1,4-benzoquinone, purity sits right up front in purchase decisions. Most suppliers advertise purity between 97% and 99% for standard applications, usually certified by high-performance liquid chromatography (HPLC) or titration methods. I've learned to compare these numbers not just with a line on a spec sheet but with the day-to-day reality of research—it can mean the difference between clear experiment results and frustrating do-overs.
Our team once switched suppliers after a string of inconsistent assay results. The culprit: a batch of quinone that barely scraped 97% purity. That impurity content crept into our experiment and forced us to repeat several trials, burning time and money. It taught me that not all “high purity” claims carry the same weight. The 99% label tends to come at a stark price jump, but in sensitive applications—organic electronics, certain syntheses, or any analytical method chasing marginal differences—the extra percent often pays for itself in reliability.
Industrial grade and lab grade aren’t interchangeable. Industrial suppliers sometimes focus on scale, sacrificing tight impurity controls to keep costs manageable. Lab-grade material earns higher trust because of stricter impurity limits, especially on metals or moisture. One supplier’s lot with purity above 98% delivered better stability and eliminated side reactions compared to a cheaper alternative. It served as a reminder: trace contaminants matter, even if they aren’t visible without a mass spectrometer.
Don’t accept vague documentation. Certificates of analysis need to match every purchase—batch numbers must line up, and the analysis should include the technique used, specific impurities identified, and values for water and heavy metals. Upper-level research and those in regulatory-heavy industries really can’t leave this to chance. It’s also worth double-checking whether the supplier offers multiple grades. High-purity options do more than inflate costs—they offer confidence that no hidden spoilers sit in the bottle.
Quality issues can snowball. Low-purity quinone might bring in trace organic or inorganic substances, nudging redox reactions off course or delivering pesky side products in polymerizations. These contaminants might not always flare up as obvious errors but can hide as lower yields and noisy data. In pharma and food applications, even tiny impurities can spell safety risks or failed compliance.
People in procurement roles can pull a few levers to make life easier for researchers and manufacturers. Build supplier relationships around transparency. Don’t be shy about asking for extra info—gas chromatography-mass spec data, in particular, can illuminate the unknowns of an off-the-shelf bottle. Develop a routine for incoming quality control sampling; random spot-checks save time later.
In my experience, the smartest teams treat purity grades as nonnegotiable, not just another product feature. The most valuable returns often show up quietly: consistent results, less troubleshooting, and the confidence to tackle tougher research questions. The story of 2-tert-Butyl-1,4-benzoquinone proves that purity isn’t a nice-to-have. It’s where real progress starts.
Anyone who has spent time in research labs knows chemicals don’t look after themselves. Get careless, cut corners with storage routines, and soon bottles fill with unwanted crystals or turn brown way too soon. 2-Tert-Butyl-1,4-Benzoquinone comes with its own rules. Ignore them, and purity drops, research budgets stretch, and your experiment ends up a mess.
I remember my first encounter with this quinone derivative: a half-used bottle, left open near a sunny window for a few weeks. Not only did it lose its deep yellow color but the results we expected went sideways until we tracked down this simple oversight. Heat, air, and light prompt this chemical to change, and that change hurts yield and results. Maintaining the right environment is not just about following a procedure—it determines data quality and, ultimately, safety.
Keep this compound dry. Moisture invites unwanted side reactions. I always count on tightly sealed amber glass bottles, kept in a desiccator. This isn’t overkill. It’s the difference between clean reactions and failed attempts. Store at room temperature, but some colleagues swear by refrigeration—just keep it out of frost zones, as extreme cold doesn’t always improve shelf life and can cause crystallization.
Sunlight degrades 2-Tert-Butyl-1,4-Benzoquinone surprisingly fast. Many labs use foil-wrapped containers or place bottles in cabinets away from stray UV light. Even room lights can slowly damage sensitive reagents. If you work in a place where labs do not prioritize these routines, losses stack up and costs surge.
Exposure to air shortens the effective shelf life. Quinones oxidize, then your working concentrations drop before you know it. I always suggest flushing containers with nitrogen before capping—standard in many research hubs but not everywhere. Those little gas bulbs or a glovebox seem like high-tech solutions at first, but over time, they protect your investment in each bottle.
Manufacturers often list shelf lives from one to two years, if you store the chemical properly. Real-world experience proves that cutting corners with storage strips away months from this number. If a lab follows best practices—low moisture, limited light, and no oxygen exposure—the compound can remain useable at expected purities for over a year. Poor storage drops it to a few months, tops.
Relying solely on dates isn’t smart. I encourage periodic quality checks, like simple melting point analysis or spectral scans. This practice often catches trouble before a ruined experiment does. If a bottle looks discolored or has solid deposits, treat that as a warning sign and don’t risk your research.
Working as a chemist, I’ve seen how basic organization saves time and budget. Staff training, written protocols, and a regular check on all sensitive chemicals increase reliability. Investing up front in the right storage containers and a dedicated space keeps everyone safer—and protects research from pointless disruptions.
Taking the time to store 2-Tert-Butyl-1,4-Benzoquinone well stands as a nod to the discipline that science demands. This habit doesn’t just guard one chemical. It builds a culture of respect for the craft, something everyone from first-year undergrads to senior researchers benefits from every single day.
| Names | |
| Preferred IUPAC name | 2-tert-Butylcyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /tuː tɜːrt ˈbɜːtaɪl wʌn fɔː ˈbɛnzoʊ kwɪnoʊn/ |
| Identifiers | |
| CAS Number | 98-29-3 |
| Beilstein Reference | 1208730 |
| ChEBI | CHEBI:52725 |
| ChEMBL | CHEMBL162970 |
| ChemSpider | 77537 |
| DrugBank | DB04244 |
| ECHA InfoCard | 19e86a94-f5f1-4992-bdae-0fea08bbac30 |
| EC Number | 208-973-1 |
| Gmelin Reference | 10460 |
| KEGG | C06702 |
| MeSH | D016696 |
| PubChem CID | 69794 |
| RTECS number | YP9625000 |
| UNII | NZD84J90BX |
| UN number | 3077 |
| Properties | |
| Chemical formula | C10H12O2 |
| Molar mass | 164.20 g/mol |
| Appearance | yellow crystalline powder |
| Odor | Odor: characteristic |
| Density | 1.14 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 1.8 |
| Vapor pressure | 2.8 Pa (25 °C) |
| Acidity (pKa) | 5.79 |
| Basicity (pKb) | 5.17 |
| Magnetic susceptibility (χ) | χ = -43.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Viscosity | 3.35 mPa·s (at 25 °C) |
| Dipole moment | 2.70 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 242.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -186.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2746 kJ/mol |
| Pharmacology | |
| ATC code | N05CM18 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P210, P280, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 108°C |
| Lethal dose or concentration | LD50 oral (rat): 1300 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (Oral, Rat) |
| NIOSH | DD3325000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Safe use for laboratory chemicals |