2-Nitrophenol has traveled a long road since chemists first documented it in the mid-1800s. As a part of the early wave of synthetic organic chemicals, it caught attention in European dye and pharmaceutical research. Back then, researchers mostly stumbled upon it while oxidizing phenol with nitric acid, trying to create new compounds with more vivid color and functionality than anything from natural sources. This compound became a frequent intermediate as the industrial revolution pushed for mass chemical production, particularly when World War periods demanded explosive precursors and pharmaceutical raw materials unlike any era before. Since then, scientists built a clear profile of its basic structure and started exploring its potential as technology, safety, and environmental awareness grew year by year.
In modern labs and factories, 2-Nitrophenol fills multiple roles—raw material, intermediate, and reference marker. At first glance, the pale yellow color might not impress, but beneath that appearance lies significant reactivity. Chemists see it as a node for creating pesticides, dyes, and flame retardants, often acting as a launchpad for more complex aromatic chemistry. Having handled countless aromatic nitro compounds, it’s easy to spot where 2-Nitrophenol stands out, bridging the practical need for cost-efficiency with pathways that let industry reach a broad range of functional molecules.
2-Nitrophenol has a melting point sitting around 45°C and tends to boil near 245°C. It crystallizes readily, giving off that signature faint phenolic, almost earthy, scent. Solubility in water sits at about 18 grams per liter at room temperature, and it dissolves even more efficiently in alcohol or ether. In solution, the compound gives a yellow color, which can deepen in alkaline conditions—a change easy to spot if you’ve ever mixed up indicator solutions for demonstration purposes. Technically, the presence of both a hydroxyl and nitro group in ortho-relationship on the aromatic ring means it displays resonance stabilization, making its chemical reactivity somewhat predictable for those comfortable with aromatic substitution and reduction chemistry. Its vapors tend to have noticeably irritating qualities, something even seasoned chemists treat with caution during weighing and transfer steps.
Industry labels typically call for minimum purities exceeding 98%, tested by melting range and chromatography. Typical packaging involves amber glass or chemically resistant polymer to maintain integrity under storage conditions. Labels carry signal words to mark its toxicity and environmental hazards—one finds the skull and crossbones on almost every bottle, followed by clear hazard statements: H302 (harmful if swallowed), H315 (causes skin irritation), H319 (eye damage risk), and H410 (acute aquatic toxicity). Lot numbers, date codes, and hazard pictograms come standard on shipments, a practice enforced by both local and international chemical safety regulations. Every reputable supplier attaches a Safety Data Sheet in the local language, clarifying emergency measures, spill procedures, and recommended storage away from oxidizers or bright sunlight.
In classic synthesis, 2-Nitrophenol starts with straightforward nitration of phenol. Mixing phenol with cold, diluted nitric acid encourages substitution at the ortho position, given its activating and directing effects. Careful temperature control (typically maintained below 50°C) keeps side-product formation in check, especially since over-nitration will throw off yields and generate unwanted byproducts like dinitrophenols. After the acid phase, separating the organic and aqueous layers becomes almost an art: proper neutralization, extraction, and recrystallization purify the main product. I’ve seen labs cut corners—like skipping double recrystallization—leading to off-spec, slightly orange-tinted material. Process engineers, especially those running kilo-scale syntheses, tend to rely on continuous flow setups to manage heat and mass transfer, achieving more predictable yields and safer operations.
The dual substituents on the aromatic ring invite further modification. The nitro group’s reducibility stands out, letting chemists transform 2-Nitrophenol into 2-aminophenol through catalytic hydrogenation or metal reduction. That conversion opens doors to dye chemistry (notably, in the production of hair colorants and textile mordants). The phenolic oxygen also undergoes etherification, esterification, and alkylation, making it a handy intermediate. In environmental chemistry, 2-Nitrophenol undergoes both photolytic breakdown and bacterial biodegradation, though these processes proceed slowly in most ambient settings. Oxidants like permanganate can cleave the aromatic ring, which scientists studying wastewater decontamination explore in detail. Because of its moderate acidity (pKa around 7.2), it forms stable salts with metal ions—a quirk that sparked interest in analytical applications during the twentieth century.
2-Nitrophenol shows up in catalogs as ortho-nitrophenol, o-nitrophenol, and the systematic IUPAC name 2-nitrophenol. Chemists may shorthand it as ONP in lab notebooks, especially during kinetic studies involving enzyme-catalyzed hydrolysis reactions (where p-nitrophenol’s yellow cousin often serves as a standard). Industrial product sheets rarely diverge from these core names, though regulatory filings sometimes include numbers from chemical inventories like CAS 88-75-5. Local names exist, but most international trade sticks with these familiar tags for clarity and regulatory compliance.
Experience teaches respect for 2-Nitrophenol’s hazards. Dermal absorption leads to methemoglobinemia—tissue hypoxia and blue coloration that signals immediate danger in exposure cases. Inhalation and ingestion can trigger headaches, dizziness, and even organ effects at higher doses. I have seen best practices demand high-efficiency fume hoods, nitrile gloves, and polycarbonate goggles, reinforced by chemical-resistant aprons in labs where acid splashes remain a risk. Spills get neutralized with sodium carbonate followed by cautious collection, as direct discharge causes aquatic toxicity. Fire risks run low, but combining the substance with strong oxidizers poses real hazards. Training programs and periodic drills back up written protocols, especially in educational or high-throughput research settings. Storage standards require locked cabinets, with clear physical separation from food, beverages, and reactive chemicals—routine for any lab or plant that uses hazardous reagents.
Agriculture turned to 2-Nitrophenol for decades as a building block for creating herbicides and fungicides, products targeting weeds and fungal pathogens that threatened crop yields through the mid-twentieth century. Pharmaceutical synthesis leans on it for specialty drugs, with the nitro or amino group anchoring side chains or aromatic scaffolds found in antihistamines and antibiotics. Dye and pigment factories shape it into colorants that appear in textiles, plastics, and sometimes inks. On the analytical front, the compound serves as a calibration standard for spectrophotometry and enzyme kinetics—something every academic lab using colorimetric assays runs into sooner or later. Environmental scientists track its fate in river systems as an indicator of industrial discharge, and some regulatory regimes list it as a priority pollutant in remediation projects involving groundwater. The variety of uses means nearly every industry that manipulates carbon rings has, at some point, found a need for 2-Nitrophenol’s reactivity and convenience.
Modern research dives into catalytic modification, bioremediation potential, and safer alternatives. Teams running combinatorial chemistry often use 2-Nitrophenol derivatives as building blocks in the search for new active pharmaceutical ingredients. Recent years brought a push for greener synthesis—microwave-assisted nitration and ion-exchange catalysis cut down on hazardous waste and energy demands. Environmental R&D crews experiment with engineered microbes capable of degrading the compound outside industrial reactors, making cleanup of contaminated soil and water more feasible on a larger scale. Toxicologists revisit exposure limits with more sensitive biomarkers, prompted by high-profile chemical spill incidents and renewed health surveillance of exposed worker populations.
Repeated studies underscore 2-Nitrophenol’s acute and chronic toxicity. In vivo animal models show the compound causes methemoglobinemia and liver dysfunction above certain thresholds. Aquatic life faces elevated risks—rainwater runoff carrying trace concentrations impacts fish, especially in egg and fry stages, disrupting development and lowering survival. Epidemiological data, though less conclusive for chronic human exposure, signals the need for vigilance in occupational settings. Efforts to determine no observed effect levels (NOELs) for differing exposure routes keep evolving, but regulators often take a precautionary stance, especially for child-bearing women and immunocompromised workers. Lab safety assessments usually integrate recommendations from both international and local agencies to form comprehensive risk mitigation plans, using actual incident data and monitoring reports as guidance.
The future for 2-Nitrophenol will likely diverge in two ways. On one hand, chemical manufacturers and green chemistry advocates continue building cleaner preparation techniques, aiming for less hazardous waste and safer intermediates. Regulations trend toward tighter restrictions on discharge and airborne exposure, with some markets demanding lower impurity levels in pharmaceutical and agricultural products. On the other hand, biotech offers hope for environmental mitigation—genetically engineered bacteria and enzymatic systems could make contaminated site remediation faster and cheaper than traditional methods. Ongoing toxicology work will shape workplace exposure limits and consumer product standards. Given its strong roots in chemical synthesis and persistent role in research, 2-Nitrophenol isn’t leaving the scene any time soon, but the ways in which scientists handle and manage it will continue to shift as knowledge, tools, and regulatory landscapes evolve.
2-Nitrophenol is a chemical name most people never hear, but it supports quite a few products and processes behind the scenes. Most folks who work with dyes, pesticides, and pharmaceuticals know this compound. My time in a college research lab, breathing in the sharp scent of nitro compounds, taught me that even the trickiest chemicals find practical uses out in the world.
Many colorants trace their roots to nitro compounds. 2-Nitrophenol is a key building block for dyes. Nearly every time you see bright fabrics or banner advertisements, something like this chemical sits deep in the background. Textile makers need vibrant colors that hold up to light, sweat, and washing. Manufacturers synthesize 2-Nitrophenol and turn it into more complex molecules, leading to that consistent red or yellow on a favorite shirt that doesn't quickly fade.
Most drug development starts with simple, reactive compounds. That’s where 2-Nitrophenol shines. It provides the basic structure for crafting medicines such as paracetamol and various antibiotics. I remember testing reactions involving aromatic nitro groups and seeing how a small difference in structure could make or break the effectiveness of a drug. Generations of chemists rely on precursors like this to build safer, more efficient medicines. Without such foundational compounds, the shelves at pharmacies would look much emptier.
Farmers and food producers depend on chemicals that push back pests without harming crops or the environment long-term. 2-Nitrophenol helps chemists produce several types of pesticides and fungicides. These compounds often require a robust molecular core—one that resists breaking down too quickly but doesn’t linger in the food chain. By starting with materials like 2-Nitrophenol, agricultural scientists design treatments that help ensure harvests remain both plentiful and safe.
Analytical chemists often reach for 2-Nitrophenol as a test substance when calibrating instruments, tracing reaction paths, or training new students. Its predictable chemical behavior makes it ideal for teaching important lab skills, especially when measuring acidity or breaking down organic compounds through oxidation and reduction. I learned titration on solutions colored with nitrophenols, spotting endpoint color shifts in the beaker. It’s a straightforward way to gain confidence in the scientific process before diving into more dangerous experiments.
2-Nitrophenol comes with risks. Exposure can irritate skin, eyes, and the respiratory tract. Countries set strict handling limits, and workplaces adopt gloves, goggles, and fume hoods for protection. Chemists and manufacturers keep careful track of how much is produced, stored, and shipped to stop accidents and environmental spills. Proper disposal is much more than a legal checkbox—it’s a public health issue. In my time handling hazardous chemicals, I learned that good labeling, robust training, and a functional ventilation system aren't bureaucratic hurdles; they're essential for keeping people safe.
Every chemical, even a humble building block like 2-Nitrophenol, calls for smart management and responsible use. As technology pushes forward, chemists look for greener synthesis routes and less toxic alternatives. Developing replacements or safer pathways can protect both workers and communities. Efficient recycling methods and tighter tracking systems close the loop, ensuring that sources of risk stay managed. Our dependence on basic chemicals won't vanish, but with sound science and well-trained people, we keep turning these silent helpers from hazards into benefits.
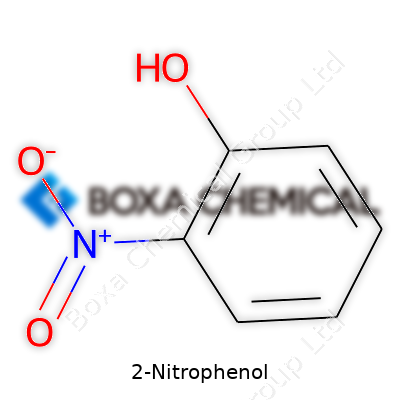
It’s easy to overlook the small stuff in chemistry, but 2-nitrophenol is a molecule with more impact than its size suggests. Its formula, C6H5NO3, reflects a specific structure where a nitro group clings to the second position of a benzene ring already holding a hydroxyl group. You’ll spot these nitro and hydroxyl groups on lab shelves and in textbooks, but their dance on the benzene ring is what makes this compound so interesting—especially to folks who work with dyes, drugs, and environmental science.
The arrangement of atoms in 2-nitrophenol isn’t random. The nitro group locks in right next to the hydroxyl, not just anywhere on the ring. That position opens up a world of chemical pathways that wouldn’t be possible if things were shuffled around. From my time teaching introductory organic chemistry, students always clicked with the “ortho” description once they built this molecule using kits: the nitro and hydroxy groups are neighbors. That physical closeness gives the molecule unique reactivity and toxicity, separating it from its isomers, like 4-nitrophenol, where the groups stand apart.
Chemists look at the practical outcomes. 2-nitrophenol pops up as an intermediate in the production of pharmaceuticals and pesticides. In research, it helps explain enzyme activity and the breakdown of pollutants. Its presence in contaminated soil or water signals industrial leaks or incomplete combustion, an important clue for environmental monitoring. The Environmental Protection Agency calls out phenolic compounds in their water quality reports, because even at low levels these molecules influence ecosystems and health.
Working with 2-nitrophenol isn’t risk-free. The nitro group boosts toxicity, while the hydroxyl increases solubility. That means it travels easily in water supplies. In labs, that calls for careful labeling, vented storage, and respect for safety protocols honed over decades. Breathing in dust or vapors can lead to irritation or more serious symptoms—this isn’t just theory, but what folks in quality control know from experience. Wastewater plants must test for this molecule regularly, often relying on high-performance liquid chromatography to spot concentrations down to the microgram. Clear data makes the difference between a safe ecosystem and one at risk.
No silver bullet takes care of pollutants alone. The chemistry behind 2-nitrophenol helps point the way. Bioremediation, using microbes that “digest” phenolic compounds, has made headway. In my graduate research group, we saw how certain bacteria break the aromatic ring and use the fragments as food. That process can turn a health hazard into carbon dioxide and water—with the right conditions, like enough oxygen and nutrients, this process moves along. Educating plant operators about these biological helpers gives them another tool beyond traditional water treatment steps.
Regulation plays its part, too. Nobody likes more paperwork, but mandated reporting and public access to contamination data put pressure on polluters and reward innovation. Open collaboration between researchers, plant managers, and regulatory agencies builds trust that outlasts any single product or process.
C6H5NO3 may fit on a page, but its effects stretch far beyond the chemistry lab. The right approach, rooted in practical know-how and honesty about risks, gets real results for people and the environment.
People working in labs or chemical industries sometimes cross paths with compounds like 2-nitrophenol. It looks like a yellowish crystal and has a sharp, biting smell. Even though it’s not shouted about as much as some other chemicals, the danger it brings is real. Studies highlight its toxic grip, especially when people breathe in its dust, get it on their skin, or swallow it by mistake.
Many folks ask if 2-nitrophenol is actually harmful. The answer is simple—yes. Its toxic reputation isn’t just talk. If it touches the skin or splash into the eyes, irritation follows. Skin may start to redden, itch, or burn. Eyes sting, water, and sometimes feel like sandpaper. The real worry comes with breathing the fumes. Even in low amounts, it causes headaches, dizziness, sick stomach, and coughing. Larger exposures pile on the risks, sometimes leading to methemoglobinemia, where blood can’t carry as much oxygen. This feels a lot like suffocating—lips or skin may turn blue, and confusion or even loss of consciousness isn’t far behind.
I’ve spent enough time around industrial settings to know safety rules shape everything in these spaces. Just a tiny spill or a dusty puff near your face can set off trouble. Chemical burns, ongoing coughs, or an odd weakness can show up later, after the job ends and boots have been hung up for the day. The CDC and EPA both mark this compound as risky, and not without reason.
The hazard doesn’t stop with people. 2-nitrophenol can flow out with wastewater, and if treatment skips a beat, it soaks into soil or trickles into rivers. Fish, insects, and birds all face the fallout. Toxic effects start showing in smaller animals fast. This chemical sticks around in the ground longer than anyone would like. Researchers found it hurts plant growth and can build up in living things. Once it moves into the food chain, it’s tough to shake loose, pointing to long-term risks most of us don’t see until harm is done.
Workplaces usually arm employees with gloves, goggles, and respirators, making exposure less likely. Strict rules from agencies like OSHA and the EPA stop this substance from floating around too freely. I’ve watched training sessions in labs save the day, stopping accidents before they get ugly. But safety slips when corners are cut or when rules are ignored. Proper disposal keeps the chemical out of the ground and water.
People have come up with cleaner production methods over the years. Some researchers look at greener alternatives or ways to quickly neutralize the compound in waste streams. Where spills happen, kits with special cleanup powders stop the mess from spreading. Combining preventative action and fast response saves workers’ health and protects local wildlife.
2-nitrophenol just isn’t something to shrug off as another dusty chemical. Each worker deserves to head home just as healthy as they arrived. With the right training, the right gear, and a respect for the risks, it’s possible to keep its hazards at bay. At the same time, cleaner, careful methods stop it from becoming an invisible threat to the world outside the factory gate.
2-Nitrophenol pops up in research labs, industry, and chemical stockrooms for a reason. It stands out as a useful building block in dyes and medicines, but it also comes with some real risks if storage drifts into sloppy territory. Sharing some experience from my own stint in a university lab, I’ve learned that ignoring storage guidance for hazardous chemicals isn’t just a paperwork problem—it becomes a safety issue for everyone in the room.
2-Nitrophenol arrives as a yellow solid with a mild, distinctive smell. It can irritate skin and lungs, but its most dangerous trait sits in its toxicity—accidental exposure, even in small amounts, poses health threats. Direct sunlight and warm rooms speed up decomposition, and spills or leaks seem minor until you spot staining and chemical burns. This isn’t a bottle anyone wants to mishandle.
Lock away 2-Nitrophenol in a cool, shaded location—think below 25°C, and definitely not on a sunlit shelf near a window. Humidity turns small storage mistakes into big ones; this powder can clump or react if moisture sneaks into the container. Tight-sealing bottles made of glass or high-grade plastics hold up best. I’ve seen labs try metal cans, but corrosion and contamination show up quicker than most expect, especially over several months.
Another task involves marking containers with easy-to-read labels and clear hazard warnings. New students and seasoned chemists alike appreciate a reminder of what lives inside those amber jars. Using chemicals without this kind of preparation gets risky—especially during late nights or tough deadlines.
Never wedge 2-Nitrophenol into a crowded cabinet with organic solvents, acids, or bases. It reacts badly with strong oxidizers or alkali, so placing it close to bleach or lye defeats the whole point of a safe chemical inventory. Color-coded shelving systems actually make sense here: they remind you in seconds what belongs together and what spells trouble.
One overlooked step involves keeping these storage spaces ventilated. Chemical fumes collect in stagnant air, making each container riskier to open. A fan or built-in vent hood cuts down on buildup, and most commercial storage cabinets deliver on this front. If the work area smells “off” or people mention burning eyes, something’s already gone wrong.
Spills happen to even the most careful folks. Written rules matter less than how folks respond. In our lab, we kept a spill kit nearby, ready for fast cleanup and disposal. Nitrile gloves, goggles, and a splash-proof apron provide the minimum gear. Scrubbing up with absorbent pads—not just paper towels—shields people and prevents spreading. Waste heads out as hazardous material, and short-cuts here invite more headaches, especially if the powder reaches drains or trash bins meant for regular garbage.
Fresh supplies arrive with documentation—check the paperwork for shelf life and disposal tips before stashing that first bottle. Manufacturers update their recommendations as new research comes in, so don’t just copy last year’s routine. With chemicals like 2-Nitrophenol, the cost of a small oversight far outweighs convenience. That’s reality for anyone working near hazardous substances, and careful storage forms the best defense against lost time, wasted money, or injury.
2-Nitrophenol stands out among the nitrophenol family. Its pale yellow crystals catch the eye, but what happens beyond the surface matters more. Working with this chemical over the years, I’ve gotten used to its stubborn, bitter scent. That smell helps experienced lab hands spot it in a room—certain whiffs never leave the memory.
This compound’s melting point sits around 61°C, just on the cooler side of many common lab chemicals. While working with it on an open bench, you’ll see those yellow crystals turn liquid in a hurry on a hot plate. The boiling point lands near 245°C, which means that in everyday labs, it rarely sees real vaporization. A fume hood and a steady hand matter because heating above the melting mark releases that heavy, choking odor.
2-Nitrophenol dissolves quite well in hot water compared to many other aromatic compounds. Add it to room temperature water and it stays mostly separate, but pour in hot water and you’ll see it disappear much quicker. In solvents like ether and chloroform, it vanishes easily. Some folks might expect organic solvents to always be better, but if you don’t use the right temperature with water, you’ll waste time and material. I learned to respect this quirk after fishing undissolved crystals out of a flask more times than I care to admit.
Anyone who’s worked with phenolic compounds notices the color difference right away. 2-Nitrophenol isn’t just a washed-out yellow; there’s a richness that’s sometimes subtle but distinctive. Its color can give you an early clue about purity. Too dark or off-shade, and odds are there’s contamination. This matters for anyone using colorimetric identification or working on syntheses where every impurity complicates things later on.
As a solid, its density hovers near 1.5 g/cm³. That helps with separation and measurement. Some laboratory errors come from not accounting for this, especially if you’re dosing powders by eye or estimating concentrations. I’ve seen new researchers misjudge amounts because they expected a lighter flake, only to end up with much more compound than planned.
2-Nitrophenol’s vapor pressure at room temperature remains low, limiting its tendency to evaporate into the air. Even in labs focused on environmental impact, its volatility rarely matches the likes of benzene. That doesn’t mean open containers are safe—a lapse in handling still fills a room with an acrid, bitter tang. Anyone using it needs decent ventilation, especially over extended work sessions.
Being a phenolic compound, it leans toward the acidic side. The nitro group intensifies this effect, allowing it to donate a hydrogen ion more readily compared to plain phenol. This impacts everything from solubility to reaction pathways. It can stain the skin yellow upon contact, serving as a quick lesson about why gloves matter. That acidic bite also gives it a certain edge in water treatment and dye industries, where reactivity gets harnessed for specific results.
Understanding physical properties shapes how chemicals get handled. Labs everywhere rely on proper melting, boiling, and solubility data to keep processes safe and efficient. By staying aware of 2-Nitrophenol’s quirks—whether that’s dealing with unexpected melting on a warm day or misjudging a concentration—chemists and industrial operators alike save time, money, and sometimes a fair bit of skin.
While routine exposure remains a concern, recognizing these properties supports safer handling. Every spilled crystal, every misplaced sample reinforces why even well-known substances deserve respect in the lab. Keeping data close and personal experience closer keeps accidents rare and productivity high, proving physical properties hold more than just academic value.
| Names | |
| Preferred IUPAC name | 2-nitrophenol |
| Other names |
o-Nitrophenol 2-Hydroxynitrobenzene ONP |
| Pronunciation | /tuːˌnaɪ.trəʊˈfiː.nɒl/ |
| Identifiers | |
| CAS Number | 88-75-5 |
| Beilstein Reference | 1209229 |
| ChEBI | CHEBI:16497 |
| ChEMBL | CHEMBL1437 |
| ChemSpider | 12040 |
| DrugBank | DB02541 |
| ECHA InfoCard | echa.europa.eu/substance-information/-/substanceinfo/100.003.388 |
| EC Number | 1.1.1.40 |
| Gmelin Reference | 57270 |
| KEGG | C01417 |
| MeSH | D009601 |
| PubChem CID | 697 |
| RTECS number | SM1400000 |
| UNII | DY6L9P641H |
| UN number | UN2517 |
| CompTox Dashboard (EPA) | WIKIID: DTXSID7020072 |
| Properties | |
| Chemical formula | C6H5NO3 |
| Molar mass | 139.11 g/mol |
| Appearance | Yellow crystalline solid |
| Odor | Odor: almond-like |
| Density | 1.198 g/cm³ |
| Solubility in water | 16 g/L |
| log P | 1.91 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 7.23 |
| Basicity (pKb) | 10.08 |
| Magnetic susceptibility (χ) | -47.9·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.621 |
| Viscosity | 1.981 cP (25°C) |
| Dipole moment | 4.23 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 104.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −35.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2139 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS06 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P264, P280, P302+P352, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 3-2-0 |
| Flash point | Flash point: 138 °C |
| Autoignition temperature | 540°C |
| Explosive limits | Explosive limits: 2.8–33% |
| Lethal dose or concentration | LD50 oral rat 299 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Nitrophenol: "299 mg/kg (oral, rat) |
| NIOSH | SN 0875 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 25 mg/L |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Nitrophenols 4-Nitrophenol 2,4-Dinitrophenol 3-Nitrophenol |