2-Methylresorcinol earned its place in the world of specialty chemicals through a steady path of industrial discovery and clever laboratory work. Chemists first isolated resorcinol derivatives back in the late nineteenth century, exploring phenolic compounds for everything from dye manufacturing to photographic developers. The addition of a methyl group to resorcinol proved to be a valuable twist, offering new reactivity and practical benefits. By the 20th century, 2-Methylresorcinol started to show up in patents for hair colorants and advanced materials, fitting right into modern chemistry's hunger for versatile building blocks. Stories from colleagues who worked in colorant labs during the 1970s remember that old tanks smelled sharply of phenolic intermediates like this one, which tells you how commonly they saw everyday use. The pace of research only picked up as pharmaceutical research grew, drawing attention to every little functional group tweak in familiar chemotypes.
2-Methylresorcinol stands as a small, specialty molecule—less famous than resorcinol itself, but no stranger to anyone working in organic synthesis or cosmetic formulation. Chemically, its skeleton is simple: a benzene ring decorated with two hydroxyls and a methyl group, occupying key positions for both hydrogen bonding and hydrophobic packing in final products. In the real world, it gets blended into hair dyes, acts as a raw material for antioxidants, and even pitches in on the sidewalls of new types of high-performance plastics. The stuff handles easily and delivers clean reactions, letting formulators dial in exactly the right properties whether coloring hair, stabilizing polymers, or building new fine chemicals. Most of the commercial product comes as a crystalline solid, easy to weigh and batch, and it rarely presents surprises on the factory floor.
Physical chemists pin down 2-Methylresorcinol as an off-white, almost needle-shaped crystalline powder, sporting a distinct phenolic aroma that wafts up the moment you crack open a bottle. Its melting point settles around 113–116°C, offering enough stability for most room-temperature manufacturing but easy enough to melt or dissolve in moderate heating. It dissolves well in organic solvents like ethanol and ether, moderately in water, and always shows strong affinity for polar protic systems—helpful for controlling its reactivity in the lab. Chemically, those two hydroxyl groups stacked on the aromatic ring make it eager for both electrophilic substitution and oxidation, with the methyl group tweaking reactivity and helping tune solubility just enough to give manufacturers more choices than with plain resorcinol.
Bottles and drums of 2-Methylresorcinol carry detailed specifications on the outside: purity by HPLC or GC tops 98%, with moisture usually less than 0.5%. Labels spell out origin, batch number, and safety data, because even seasoned users know that phenolics deserve respect. Modern regulatory frameworks in the US, EU, and Asia set tight tolerances for trace contaminants—like heavy metals, residual solvents, and polychlorinated impurities—since this material often ends up in consumer products. Producers provide tech sheets covering spectral data, recommended storage between 2–8°C, and suggested shelf life, but seasoned chemical handlers always check for color shifts and clumping anyway. Everyone in the supply chain keeps paperwork squared away for safety, especially since regulatory spot checks can trigger expensive recalls.
Industrial synthesis of 2-Methylresorcinol commonly starts with 2-methylphenol (o-cresol), which chemists subject to hydroxylation reactions under rather focused conditions. Catalysts like iron salts and oxidants such as hydrogen peroxide or atmospheric oxygen coax the cresol ring into picking up hydroxyl groups at the right spot, letting manufacturers steer product ratios toward the target compound. Some factories have leaned into newer enzymatic approaches, seeking cleaner by-products and lower environmental impact, but classic chemical methods still dominate at scale. Once the crude product drops out, purification—often via recrystallization from methanol or water—brings the final solids to market-spec purity, ready for use in downstream chemical transformations. In research labs, similar reactions run on a smaller scale, with students learning practical organic techniques the old-fashioned way.
2-Methylresorcinol opens the door to plenty of downstream transformations. The hydroxyl groups invite etherification, esterification, and coupling with diazonium salts—a big reason why this molecule’s fingerprints show up in azo dyes and pharmaceutical intermediates. Researchers often methylate or acetylate it to tweak solubility, or they fuse it into larger rings in the search for new bioactive chemotypes. Chemical modifications bring a practical dimension, letting industrial partners tune thermal stability or tweak electronic properties just enough for a plethora of applications in electronics and organic synthesis. One memorable afternoon in grad school, I watched our group use a gentle oxidation to dial down color body formation in hair dye cocktails, saving a production run from expensive discoloration and proving that just a little chemistry goes a long way.
2-Methylresorcinol picks up an alphabet soup of names on suppliers’ catalogs and regulatory lists. Its IUPAC name stands as 2-methylbenzene-1,3-diol, but you’ll find it listed as 4-methylresorcinol, 2-hydroxy-5-methylphenol, or even C.I. 2-Methylresorcinol in old dye industry reports. International trade assigns it a CAS number—respected by customs officers and regulatory watchdogs alike—as yet another way to pin down its identity across jurisdictions. On product shelves, it may bear a proprietary trade name if blended into a formulated product, reminding users to cross-check safety data and avoid confusion with similar-sounding chemicals that have very different hazards or functions.
Anyone who handles 2-Methylresorcinol learns to respect phenolic compounds’ bite. Skin contact causes irritation and sometimes allergic responses, especially among workers exposed over long shifts. Inhalation of dust or aerosolized particles risks respiratory discomfort, so proper masks and local exhaust ventilation matter as much as gloves and goggles. Factories and research labs post clear signage: avoid open flames, monitor indoor air, practice quick clean-up of spilled material. Health and safety training keeps mistakes rare, but even seasoned chemists keep the emergency shower unblocked and neighbors aware. Disposal regulations in every major economy classify phenolic waste for incineration or controlled landfill, keeping ground and water safe.
2-Methylresorcinol finds its main stage in the cosmetics industry, where it anchors certain oxidative hair dyes. The molecule brings deep shades reliably and resists premature fading, pleasing chemists and stylists alike. Its chemical flexibility also supports research in pharmaceuticals: intermediate compounds, antioxidant building blocks, or even links in the chains of future drug candidates. Polymeric chemists pull it into the world of high-end plastics and resins, counting on its heat stability and subtle electronic tweaks. In dye and pigment labs, it remains an old standby, appreciated for its cost-effectiveness and sure performance, especially now that demand for new color spaces rises from both fashion and industrial markets.
R&D teams continue to push 2-Methylresorcinol into new territory. One trend focuses on developing greener manufacturing routes—using biocatalysts, milder oxidants, or energy-saving process design—because traditional synthesis sometimes leaves behind too much waste. University labs and corporate partners collaborate on computational modeling, using quantum chemistry to predict reactivity in new dye cocktails or pharmaceutical intermediates. Advances in analytical detection—think high-res mass spectrometry—help spot trace impurities, keeping products within the ever-tightening regulatory limits pushed by health agencies. My own experience in a specialty chemicals start-up showed just how hungry the field remains for even tiny molecule tweaks; we chased bio-based substitutions looking for patents, but always returned to well-understood phenolic scaffolds like this one to bridge the gap.
Scientists keep a close eye on the toxicology of 2-Methylresorcinol, especially since much of it ends up in consumer-facing goods. Animal studies show low-to-moderate acute toxicity, with irritation to skin and eyes as the main concern at expected exposure levels. Chronic exposure data remains limited, but repeated occupational contact raises the risk of sensitization. Regulatory reviews—especially in Europe under REACH and in the US via the EPA—push manufacturers to test for genotoxicity, endocrine disruption, and environmental persistence. In recent years, cosmetic ingredient panels have set conservative thresholds for inclusion in finished products, spelling out application restrictions and recommended rinsing instructions. Data on aquatic toxicity supports guarded waste treatment protocols, reminding all users to minimize environmental release.
Future directions for 2-Methylresorcinol look busy. Demand from the hair dye sector keeps holding steady, and any shift toward bio-based or greener consumer products puts pressure on suppliers to improve synthetic routes, guarantee ultra-high purity, and trim waste at every step. Strong ties to pharmaceutical R&D keep opening up possibilities for new patents and lifecycle management of aging drug classes, since subtle functional group changes like a methyl group often drive patentable advances. As analytical and computational tools improve, formulators get better at predicting all the subtle performance tweaks possible with ring-modified resorcinols, feeding innovation in dyes, plastics, and fine chemicals. If industry and regulators work hand in hand on safety testing and exposure monitoring, 2-Methylresorcinol ought to keep its reputation as a reliable, hardworking chemical player for years to come.
2-Methylresorcinol pops up far less in daily chatter than ingredients like vitamin C or caffeine, but it’s tucked away in plenty of products at home and at the store. In my experience talking with folks working in haircare, it comes up most during discussions about permanent and semi-permanent hair dyes. Known for helping create vibrant, lasting color, this little molecule has become part of the beauty toolkit and shows up in salon formulas that claim to work wonders for grey coverage or bold transformations.
Many kinds of hair dyes achieve their magic by triggering chemical reactions inside the hair shaft. 2-Methylresorcinol acts as a primary intermediate, reacting with oxidizing agents like hydrogen peroxide. This reaction produces pigmented molecules that latch onto the hair, creating shades ranging from auburn to deep brown. Years ago, I watched stylists debate which molecules gave the richest tones, and 2-Methylresorcinol often won out for depth and reliability. Science backs this up, as dye cosmetic chemists look for molecules that won’t wash out quickly or fade in sunlight.
Walk into a chemistry teaching lab and you might spot 2-Methylresorcinol in reagent bottles. It’s used to help test for sugars or create specialty plastics and adhesives. Sometimes, I’ve heard of it being used as a building block when synthesizing more complex molecules in industry. Since it’s a phenolic compound, chemists value how reactive – and versatile – it can be when piecing together more functional substances. Even though its presence in daily items beyond hair dye isn’t often advertised, it plays a quiet but important backstage role.
Regular conversations with dermatologists and toxicologists surface one issue again and again: any ingredient used on skin or hair has to pass safety checks. Studies show 2-Methylresorcinol doesn’t top the toxicity charts, but, just like with other hair dye chemicals, it can trigger allergic reactions in a small percentage of people. Red, itchy scalps or rashes signal a sensitivity. The European Commission has guidelines capping its concentration in hair products – not because it’s especially dangerous, but to manage the risks of chronic or repeated exposure.
Digging through research, concerns center mostly on possible skin irritation, especially for those with already sensitive skin. While allergic reactions seem rare, patch testing remains common sense before any hair dye job. I remember chatting with salon owners who always demanded allergy tests for new clients, especially after a couple of customers ended up with red patches behind their ears after coloring.
People are pushing for safer, more sustainable alternatives in beauty and chemistry. Companies now explore plant-based and low-allergen dyes, though synthetic ingredients like 2-Methylresorcinol still deliver strong, predictable results. The challenge: new, “natural” compounds don’t always deliver lasting color or coverage. Science edges forward but hasn’t fully replaced the old standbys yet.
For people who color their hair often, reading every label and asking your stylist about ingredients pays off. If a product causes irritation, share feedback with companies—it encourages better and safer formulations for everyone. We deserve beauty and self-expression that don’t come at the expense of health.
2-Methylresorcinol pops up in the ingredient list for box dyes, salon color creams, and a few permanent hair treatments. Some folks spot it on labels and get nervous, mostly because science names in cosmetics always look intimidating. The real question centers on whether this compound belongs anywhere near your scalp or skin.
As someone who values science-backed choices, I wanted to know what reputable sources have to say. Cosmetic chemists include 2-Methylresorcinol for its magic in fixing color deep inside the hair shaft. It helps the color last longer and look rich. The European Scientific Committee on Consumer Safety (SCCS) took a good look at this compound. They found it safe in permanent hair dye and eyebrow dye, but only if the limit in the mixture keeps to 1.8%. That threshold matters, because strong chemicals can hurt the skin in larger amounts.
The main worry deals with allergic reactions and skin irritation, especially with frequent coloring or sensitive skin. Out of curiosity, I checked patch test studies. These studies say allergy risk with 2-Methylresorcinol stays low, but not zero. Patch tests rarely show a reaction in people with no history of dye allergies. Most rashes or itching seem to happen with multiple colorant chemicals piled together, not this ingredient on its own.
Still, dermatologists I’ve talked with urge extra caution for anyone who has eczema or past allergies to hair dye. They remind clients to patch test on a small area before every use. This habit won’t guarantee safety, but it can prevent nasty surprises.
Concerns over hair dye rarely stop at skin safety. People ask about waste water and what these chemicals leave behind in the environment. So far, data on 2-Methylresorcinol’s impact after rinsing down drains stays pretty thin. The bigger hazard seems to come from how dyes and chemicals as a group interact with ecosystems, not one ingredient alone. More research in this area could nudge the beauty industry to develop cleaner, greener choices.
To lower risk, everyone benefits from reading product labels and checking for professional certifications. Brands that publish their ingredient origins and test results make it easier to trust what goes onto your skin. For people with no allergy risks but a history of scalp irritation, switching to ammonia-free dyes or plant-based alternatives might help. I’ve seen more friends go that route, especially as natural beauty trends grow.
Regulators watch these ingredients, so laws already block companies from using unsafe concentrations. If more people highlight their experiences and demand greater transparency, manufacturers get nudged toward safer formulas. The dream should be hair color that pops and shines without nagging health questions or complicated chemical jargon.
2-Methylresorcinol may sound technical, but for those mixing hair dyes or working in a lab, its safety should never get pushed aside. This chemical has found its spot in research spaces and the cosmetic world, showing up in hair colorants for years. With its benefits also come real risks—mainly the fact it’s heat sensitive and can cause irritation if mistakes happen. Everyone deserves protection, whether at home or in a research facility.
People try to save space and tuck containers wherever it seems convenient. I once saw a colleague pop a bottle right next to a sunny window, and over the weekend, a sticky mess waited for us on Monday—light and heat broke down the compound, giving us a stink and extra work. Mishandling can lead to degradation, and degraded chemicals don’t just lose effectiveness—sometimes they turn more hazardous.
Store 2-Methylresorcinol in a cool, dry spot. Temperatures above room level push the risk higher—chemical breakdown speeds up, or the substance clumps. I’ve learned to keep similar compounds below 25°C (77°F), and if it’s a larger stash or in an unpredictable climate, a refrigerator works best. Just don’t freeze it, as this can mess with consistency and packaging.
Moisture becomes an enemy too. Humidity can wreck a powder’s stability—clumping or reacting with water vapor, even inside a closed cabinet. Silica gel packs pull their weight, ensuring the inside stays bone dry. If using the chemical in a humid city or basement, it’s worth adding those moisture absorbers.
Keep containers sealed tight and away from direct sunlight. Light often jumpstarts chemical changes, sometimes unseen until it’s too late. Amber glass bottles or opaque containers shield the contents—there’s a reason pharmacies use them for sensitive meds too. Oxygen creeping in can also cause subtle shifts; always position lids tightly, and swap out damaged caps instead of taping them up.
Mislabeling or skipping dates opens the door to confusion. One of the smartest habits I picked up from a senior chemist was labeling each bottle with the date opened. No guessing games on age or stability. Handling chemicals “blind” wastes supplies and puts people at risk.
It’s easy to overlook simple checks when daily routines get busy. Unlabeled bottles build up on shelves, and soon nobody knows what’s safe or spoiled. I’ve watched good chemicals go bad because of “just for today” shortcuts—soon leading to costly cleanups or even health scares. Regular checks, and quick removal of anything questionable, prevent accidents. Simple habits like storing chemicals up high, far from kids or pets, close loopholes that curiosity or carelessness might exploit.
It isn’t about strict rules for the sake of rules. Every small storage step makes workplaces and homes safer. Use original containers, keep up with inspection schedules, lean on moisture absorbers, and trust protective packaging. Reputable suppliers usually include storage advice on their labels—following these helps keep supplies from turning dangerous.
The more we treat 2-Methylresorcinol (and every chemical) with respect in storage, the fewer accidents pop up. Experience shows that the right habits make all the difference—protecting work, products, and most importantly, people.
I remember the first time reading an ingredient label and spotting something like “2-Methylresorcinol.” Folks with an allergy to hair products probably recognize it, since it pops up in hair dyes pretty often. It’s a chemical, sure, but so is caffeine, so are various ingredients we hardly think about. The question doesn’t boil down to the name on the label. What matters is how this ingredient interacts with people, and how the industries using it manage those risks.
2-Methylresorcinol appears mostly in cosmetics—especially hair dye formulas—because of its coloring properties. Once someone hears “chemical” and “hair dye,” the mind fills with images of all sorts of hazards. Bodies like the European Chemicals Agency (ECHA) and U.S. Environmental Protection Agency have dug into its profile. On the skin, this stuff causes irritation for some people, especially if they're prone to allergies. Users with sensitive skin complain of rashes, redness, or itching. Animal studies suggest at high concentrations, it irritates the eyes as well.
Toxicity takes on different forms. Chronic exposure to high levels causes trouble, but typical consumers will barely approach those kinds of conditions. Salon workers, though, stand closer to risk, handling dyes on a daily basis or working in less-ventilated spaces. Prolonged skin contact or inhaling vapors can rack up the risk for them. Respiratory symptoms and headaches become possible, and for those with asthma, things get more complicated.
Researchers have published safety studies over the years. According to a 2022 summary in the journal “Contact Dermatitis,” a small share of users reacted with allergies, but most folks tolerated moderate levels fine. It doesn’t show strong links to cancer, reproductive harm, or DNA mutations at concentrations used in hair dye. Safety boards in the EU, U.S., and Japan review the data every few years, updating guidelines and reviewing whether maximum allowable concentrations change.
Regulators don’t just rely on company claims. They require manufacturers to submit toxicology reports. In Europe, only a strict percentage of 2-methylresorcinol gets allowed in products—there’s a reason for those limits. The limits come from lab data and trends showing what causes trouble, and authorities check up on those numbers regularly. So, only low levels, usually under 2%, land in everyday cosmetics.
People dealing with skin conditions or allergies probably know the drill: patch testing products or scanning ingredient lists. There’s room for better transparency, too. Full and accurate labeling helps people minimize unnecessary risk. Education goes a longer way than blanket bans. Employers in salons and factories can make a difference by providing gloves, ventilation, and safe handling instructions to lower workplace exposure.
Alternatives are out there for people aiming to clear their routine of potential allergens. Plant-based or mineral colorants offer options, but they don’t always match performance. Research labs tinker with new molecules trying to strike a balance between safety and effectiveness.
Ignoring concerns isn't wise, but demonizing chemicals without reading data keeps nobody safe. Informed choices depend on good evidence and clear communication. My experience tells me people want simple answers and honest options. For now, the best call is to stay aware, read labels, and keep an eye on authoritative health updates—especially if you notice reactions or work in an environment where exposure could climb.
The relevance of knowing the chemical structure and formula of 2-Methylresorcinol reaches well beyond chemistry labs. This compound plays a noticeable role in hair dye formulations, where safe and reliable ingredients matter. Its molecular formula, C7H8O2, tells us about seven carbon atoms, eight hydrogens, and two oxygens arranged in a particular way. The magic, though, is in the details—the way these pieces fit together shapes its properties and how it can be used.
Anyone who's cracked open an organic chemistry textbook will remember how a benzene ring forms the backbone for many familiar chemicals. That flat ring of six carbons holds its place at the center of 2-Methylresorcinol. Two hydroxyl (–OH) groups attach at the 1 and 3 positions. The extra methyl (–CH3) group connects at position 2. For folks drawing this on paper, the rings start to look like a clock—hydroxyl groups at "1" and "3," methyl at "2." That pattern defines its identity.
Visualizing chemicals in three-dimensional space brings new appreciation for why they work in real-world applications. That arrangement gives 2-Methylresorcinol its unique mix of solubility and reactivity—traits that manufacturers consider from the earliest stages of product design.
The details of structure spill over into practical life. In semipermanent hair coloring products, 2-Methylresorcinol helps develop shades that last but stay gentle enough for repeated use. Its specific architecture mellowed out the harshness seen with some color precursors, making it a safer alternative compared to older dye intermediates. That’s no small feat—skin irritation and allergic reactions remain common concerns with many hair dye ingredients.
Consumer safety reviews from agencies like the European Scientific Committee on Consumer Safety show that 2-Methylresorcinol, at recommended concentrations, maintains a solid safety profile. Its presence in hair dye formulas comes from years of ingredient testing and reformulation, all aimed at balancing effectiveness with reducing risk.
Those reading product labels get power from knowing what’s inside the bottle. Understanding that C7H8O2 isn't just a random string but a carefully chosen building block encourages more informed decisions. Health professionals and regulators use this kind of data to set exposure limits and investigate ingredient safety claims. Seeing the structure helps connect the dots between chemical theory and the lived experience of people wanting safe, vibrant hair color.
Some issues come from improper formulation or not following recommended concentrations. Quality control and transparency matter—a trusted supply chain and full ingredient disclosures help prevent counterfeit products from causing harm. Scientific transparency, not just for regulatory compliance but for the end-user’s peace of mind, keeps 2-Methylresorcinol’s reputation clean.
Simple actions can limit risks further: patch testing for allergies, reading product instructions, supporting brands that publish safety data. On the manufacturing side, researchers continue to study the reactivity of 2-Methylresorcinol with other dye ingredients, seeking out combinations that work well without unexpected byproducts.
The story of 2-Methylresorcinol represents a patch of common ground between consumers, scientists, and businesses. Looking at its structure and formula opens up conversations about trust, evidence, and how even tiny changes in molecular design can have ripple effects in everyday life.
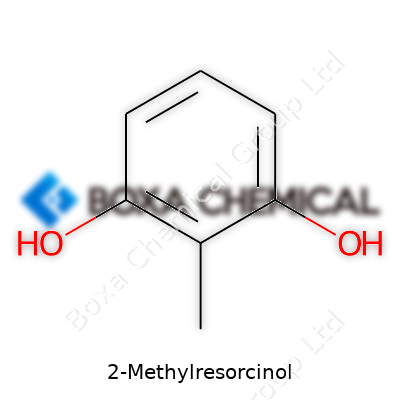
| Names | |
| Preferred IUPAC name | 2-methylbenzene-1,3-diol |
| Other names |
2-Methylresorcinol 2-Methyl-1,3-benzenediol 2-Methyl-1,3-dihydroxybenzene 2-Hydroxy-5-methylphenol 2-Methylresorcin |
| Pronunciation | /tuː ˌmɛθɪl rɪˈzɔːrsɪnɒl/ |
| Identifiers | |
| CAS Number | 608-25-3 |
| Beilstein Reference | 1310713 |
| ChEBI | CHEBI:17219 |
| ChEMBL | CHEMBL3184827 |
| ChemSpider | 126666 |
| DrugBank | DB11233 |
| ECHA InfoCard | ECHA InfoCard: 100.006.924 |
| EC Number | 205-255-4 |
| Gmelin Reference | 66080 |
| KEGG | C01735 |
| MeSH | D016716 |
| PubChem CID | 13609 |
| RTECS number | DH8225000 |
| UNII | D3PWH8C5WL |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C7H8O2 |
| Molar mass | 124.14 g/mol |
| Appearance | White to beige crystals or powder |
| Odor | Odorless |
| Density | 1.102 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.88 |
| Vapor pressure | 0.001 hPa (20 °C) |
| Acidity (pKa) | 9.46 |
| Basicity (pKb) | 8.64 |
| Magnetic susceptibility (χ) | -50.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.583 |
| Viscosity | 1.161 cP (25°C) |
| Dipole moment | 1.63 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 165 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -262.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3462.7 kJ/mol |
| Pharmacology | |
| ATC code | D11AE06 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | Precautionary statements: "P261, P280, P302+P352, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 140°C |
| Autoignition temperature | 545°C |
| Lethal dose or concentration | LD50 (oral, rat): 800 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Methylresorcinol: "475 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1% |
| Related compounds | |
| Related compounds |
Resorcinol 4-Methylresorcinol 2-Ethylresorcinol Catechol |