Chemists first synthesized 2-Methylanthraquinone in the late 19th century, during a time when aromatic compounds changed the face of industry and colored the modern world. Back then, researchers dug deep into coal tar, finding that by tweaking the anthraquinone molecule, they could tune its properties for dyes, pigments, and other uses. German dye houses, hungry for new shades, pushed the boundaries with methylated derivatives, and 2-methylanthraquinone quickly found a place in synthetic dye development. Over time, it expanded out of textile dyes, making inroads into organic synthesis and chemical engineering.
2-Methylanthraquinone rolls off the tongue easier once you’ve handled it in a lab or on a production line. Chemists and industries know it by a few other names—beta-methylanthraquinone, and sometimes, with a nod to the systematic approach, as 2-methyl-9,10-anthraquinone. The solid itself sports a yellowish crystal appearance, sometimes packed in bags for industrial distribution. While it began its journey in the dye sector, markets now pull it into pulp bleaching, chemical intermediates, and certain specialty pharmaceuticals.
At room temperature, 2-methylanthraquinone shows up as yellow to orange crystalline flakes with a faint aromatic odor that reminds some chemists of vintage dye factories. It measures a melting point near 175°C and pushes its boiling point well beyond what most organic syntheses require, over 390°C, limiting volatilization during reactions. Its density hovers around 1.32 g/cm³ and it resists dissolving in water—though it finds a home in organic solvents like chloroform and acetone. Chemists value its stable quinone core and the way the methyl group at the 2-position tweaks both solubility and reactivity, making it a favorite for tailored modifications.
Industry sets the minimum purity at around 98% for research-grade material, while large manufacturers watch for trace contaminants like anthraquinone and polymethyl anthraquinones. Suppliers label the product with hazard pictograms, clear batch numbers, and shelf-life advisories. Safety data sheets detail the compound’s reactivity profile, storage conditions—such as dry, cool, and ventilated spaces—and recommended PPE. Every drum or bottle carries information in compliance with the Globally Harmonized System (GHS), using CAS No. 84-54-8 to guarantee traceability and quality.
The factory method for making 2-methylanthraquinone starts with toluene or methyl-substituted naphthalene, carefully oxidized with reagents like chromic acid or through catalytic air oxidation. Chemical engineers learned to feed the correct ratios of raw materials, moderate temperatures around 180–220°C, and control oxygen flow rates. Crystallization from the reaction broth, followed by washing and drying, leads to a pure, free-flowing powder suited for downstream use. Research sites running smaller batches sometimes favor a Friedel–Crafts acylation approach, letting them test modifications without investing in massive infrastructure.
Chemists see 2-methylanthraquinone as a versatile starting block. Functionalization of the methyl group unlocks new derivatives. Oxidizing that methyl group leads to carboxylic acids, while nitration or sulfonation at the available ring positions allows access to specialty pigments and intermediates. Basic reduction yields methylanthrone, prized for redox catalyst testing. Its stable structure, yet reactive sites, make it useful for advanced organic synthesis, including pharmaceuticals, agrochemicals, and high-performance dyes for electronics.
Across markets, 2-methylanthraquinone takes on various labels: beta-methylanthraquinone, 2-methyl-9,10-anthraquinone, and sometimes the shorthand MAQ. Chemical registries often stick with the IUPAC name for clarity. In commercial trade, manufacturers highlight the methyl position to avoid confusion with other isomers. Catalogues from Sigma-Aldrich to local Chinese suppliers refer to the same robust yellowish solid—just under a different brand or language.
Handling this compound safely calls for experience with quinones. Staff loading reactors wear gloves, goggles, and lab coats, especially since the dust can irritate skin and eyes. Dust extraction or local exhaust helps prevent respiratory exposure. Companies follow Occupational Safety and Health Administration (OSHA) guidelines in the US and reach similar standards overseas, monitoring both air quality and waste disposal practices. Emergency protocols demand eyewash stations and spill kits, prioritizing prevention and swift response during bulk handling.
Papermaking industries prize 2-methylanthraquinone for its efficiency in the soda pulping process. By catalyzing lignin breakdown, it slashes chemical consumption and boosts pulp yield, which appeals to both environmental and economic priorities. Synthetic dye manufacturers use it to generate deep shades in wool and nylon, appreciating the fastness and intensity it lends to final products. In organic chemistry labs, this compound lays the groundwork for more complex molecules, cropping up in drug research and as a redox mediator for electrocatalysis.
Scientists look for new catalysts, drugs, and functional materials using 2-methylanthraquinone’s core as a launchpad. Analytical chemists have mapped its UV–Vis absorption profile, using it in photochemical projects and dye-sensitized solar cells. Biochemists test methylated anthraquinones for anticancer and antioxidant activities, hoping to separate unique biological actions from close relatives like anthraquinone or emodin. Global research efforts have turned this compound into a tool for probing electron transfer, a hot topic in green chemistry and alternative energy.
Toxicologists ran a battery of tests on 2-methylanthraquinone, comparing it to classic anthraquinones used in dyes and pigments. Rodent studies revealed low oral acute toxicity, but researchers still warn about chronic exposure due to potential kidney and liver stress. Carcinogenicity testing on the pure compound gave mixed results—with some studies showing minimal evidence and others pushing for more data. Workplace safety programs measure airborne particles, while municipal regulators limit emissions in wastewater, since quinones after long exposure can show up in river sediment and food chains.
Green chemistry demands more from established chemicals; 2-methylanthraquinone has a chance to become a linchpin in sustainable industry. Papermills already save energy and cut emissions using it, but fresh attention from battery scientists and medical researchers might unlock game-changing applications. With the right partnerships between manufacturers, academics, and environmental watchdogs, cleaner synthesis routes and inventive functionalization could expand both its range and safety profile. In the years ahead, expect production to shift toward eco-friendly processes and regulation to push for safer practices across supply chains, creating new benchmarks for chemical responsibility.
Walk through any paper mill, and someone will point out a funny yellow compound helping wood chips shed their identity and turn into something new. That’s 2-Methylanthraquinone. For decades, this yellow crystal with the unwieldy name has helped paper producers pull more fiber out of wood and deal with less waste. Any conversation about efficient forest use circles back to chemicals like this. It may sound niche, but the footprint of this compound stretches farther than most folks realize.
Pulp mills tackle mountains of timber and need a way to separate useful cellulose from tough lignin. Mills adding 2-Methylanthraquinone to the pulping process find they get stronger pulp and better output from the same trees. Rather than burning through more wood, they boost what comes out of every log. This helps reduce pressure on forests, which matters to people like me who grew up around logging towns and saw how clear-cutting can cut families’ futures short, not just trees.
Every chemical in industry raises questions: Will it harm workers? Does it build up in the rivers or end up in tap water? In my experience, those questions travel hand in hand with stories about production. With 2-Methylanthraquinone, research points to a fairly low toxicity for humans when handled right. Rigorous safety guidelines push companies to follow strict storage, ventilation, and handling rules. The compound doesn’t stick around in the environment like notorious industrial chemicals have. That’s key for anyone thinking about long-term health, from the folks running the machines to families near the plant.
The chemical puzzle of 2-Methylanthraquinone doesn’t end with pulping. It also chips in as an intermediate in dye manufacturing. Picture bright fabrics or colored plastics — without stable intermediates, lots of color products would fade or fail. What turns heads these days is its role in organic chemistry research, including battery and solar cell design. Scientists keep searching for sturdy, affordable molecules to build sustainable tech, and this compound pops up in their experiments. The research is ongoing, and results look promising, especially in flow batteries where steady performance over many cycles makes all the difference.
People keep asking if greener options exist. Some mills look for other pulping agents, but giving up yield or product strength rarely flies in board rooms. Investment in better recovery systems can help reduce any losses to wastewater, which matters for both the bottom line and clean rivers. Ongoing research into biodegradable or even naturally-derived alternatives continues, but for now, compounds like 2-Methylanthraquinone dominate because they work so well. Regulatory agencies ought to push for transparency, tight discharge limits, and open reporting, so that people living near plants or working inside them stay protected.
Real progress rarely comes in one dramatic leap — it tends to happen in small steps, with a lot of debate, trial, and focused research. 2-Methylanthraquinone’s story is all about that push for efficiency and safety, and it’s worth watching as industries and communities learn from each cycle of use and improvement.
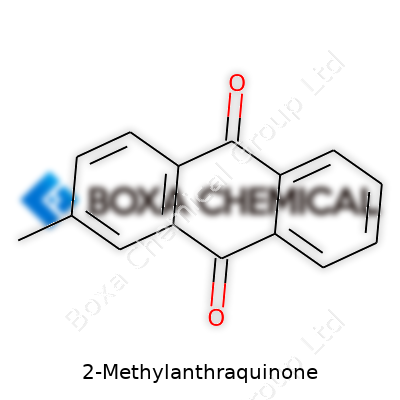
The chemical landscape often seems crowded and complex, but learning about something as specific as 2-Methylanthraquinone can reveal the everyday connections between science and products we rely on. The molecular formula for 2-Methylanthraquinone is C15H10O2. This formula means the molecule has fifteen carbon atoms, ten hydrogen atoms, and two oxygen atoms. The name gives away a lot: it’s a derivative of anthraquinone, only with a methyl group (-CH3) attached at the second position of the anthraquinone skeleton.
2-Methylanthraquinone features three benzene rings fused in a linear arrangement—an unmistakable feature in polycyclic aromatic hydrocarbons. At the core, two carbonyl groups (C=O) anchor themselves at the 9 and 10 positions of the anthracene backbone. The methyl group finds its spot at the 2-position, changing the way the molecule behaves chemically and physically. Visualizing this structure helps explain the molecule’s reactivity and its uses.
Years ago working in an industrial lab, I saw 2-Methylanthraquinone in action during the pulping of wood. For many people, the molecule’s main claim to fame sits in the field of papermaking, as it plays a part in the anthraquinone process used to pulp plant material. The methyl group boosts the efficiency of anthraquinone, making it more effective at breaking down wood chips, which saves both time and energy in large operations. More efficient pulping translates into less waste and lower costs, and that trickles down into sustainability goals for big papermakers.
The arrangement of those rings, carbonyl groups, and the methyl pendant might look unremarkable in a textbook diagram, but it brings real advantages. 2-Methylanthraquinone shows enhanced solubility and reactivity over its parent compound. In synthesis labs, the molecule acts as a stepping stone to make dyes, pigments, and even pharmaceuticals. The tweaking at the 2-position offers a road for chemists to alter properties, tailoring new materials for color fastness, heat resilience, or specific interactions with biological molecules.
We see the power of molecular fine-tuning in environmental science, too. With every additional methyl group on molecules like anthraquinone, toxicity, bioaccumulation, and breakdown pathways shift in unpredictable ways. Regulatory agencies and research teams keep a watchful eye on these compounds, especially those that make their way into soil and water streams after industrial use. It is important for manufacturers and researchers to stay updated with peer-reviewed studies and government guidelines, since small changes on a chemical skeleton can create big challenges in terms of remediation or safe disposal.
Responsible chemistry demands attention to more than just the product’s immediate function. Improving the safety profile, reducing side reactions, and finding more environmentally friendly alternatives continue to drive research into compounds like 2-Methylanthraquinone. Methods such as greener synthesis, improved recycling, and waste management can offset some of the downsides, especially if paired with transparent communication between scientists, industry, and the public. The next time you pick up a paperback or see a vivid textile, there’s a decent chance the chemical structure of 2-Methylanthraquinone played its part somewhere in that story.
Everyone likes to trust that chemicals used in products and industries today won’t put our health or the environment at risk. 2-Methylanthraquinone, a yellowish solid, finds use mostly in the papermaking world—helping break down wood chips in pulp production—plus some roles in dye and pigment manufacturing. As someone who has spent years paying attention to workplace safety discussions, chemical labels, and databases, I know that chemical safety isn’t just something for specialists to worry about; it affects anybody whose job, neighborhood, or water supply touches these substances.
Digging up research from the past decade, there’s not a ton of public panic around 2-methylanthraquinone, especially compared to notorious hazards like lead or benzene. Still, low profile doesn’t mean risk-free. The European Chemicals Agency gives the chemical an official tag as “harmful if swallowed” and “harmful to aquatic life with long lasting effects.” Industrial safety data sheets agree, urging gloves, goggles, and good ventilation since swallowing or inhaling the dust may upset your gut, skin, or lungs.
Toxicity studies on animals have flagged things like mild liver or kidney changes after persistent, fairly high doses. Human data looks thin, but the absence of evidence doesn’t guarantee safety; it usually points to a lack of comprehensive research. I remember seeing the jump in attention paid to seemingly benign substances before—only to find out, years down the line, that regulations needed tightening. So “not obviously deadly” is not the same as safe for long-term or widespread contact.
People sitting in offices rarely bump into 2-methylanthraquinone. Those working on paper mill floors or mixing pigments run a bigger risk, simply because they handle or breathe the stuff. The dust floats around if controls are sloppy, and accidents happen, whether from poor training or neglected maintenance. Waterways near some facilities can pick up chemical traces, threatening aquatic life. Small doses kill algae, shellfish, and plankton in lab experiments. Those might not headline the evening news, but that’s where food chains can start to unravel.
This reminds me of times colleagues brushed off “just dust” as harmless—until chronic coughs or dermatitis became the norm at work. Keeping tabs on even lesser-known chemicals pays off. No one wants to relive the mistakes made with PCBs or asbestos, where a slow trickle of problems grew into a tide no one could ignore.
Companies using 2-methylanthraquinone benefit most from treating it with respect. Effective extraction fans, good housekeeping, and easy-to-understand training protect lungs, skin, and the next generation of workers. Personal protective equipment—proper gloves and masks—should not be “optional on hot days.” Installing real-time air monitors, doing regular medical checks, and mopping up spills without delay make practical sense.
Communities near river outfalls also need solid regulation. Wastewater treatment designed for persistent organics, plus transparent reporting to neighbors, cuts down risk and builds trust. Engineers and regulators often talk up “green chemistry,” swapping older chemicals for newer, less toxic alternatives—so keeping pressure on manufacturers to innovate can push the whole sector in a safer direction.
2-Methylanthraquinone doesn't call for hand-wringing, but treating it as boring background noise would sell workers and neighbors short. If the past tells us anything, it's that a little vigilance beats a lot of clean-up.
Working with chemicals like 2-Methylanthraquinone sounds routine for anyone familiar with dyes, paper-making, or organic synthesis. But the familiar can turn risky if it’s taken for granted. Nobody wants a ruined experiment, or worse, an accident that harms a colleague or contaminates the workspace. Experience shows that the folks who treat chemicals with consistent care keep both their work and their health intact.
2-Methylanthraquinone brings moderate hazards to the table. Breathing in dust or getting the solid on your skin causes irritation. Prolonged or repeated exposure increases those risks, and that’s not a footnote anyone wants to discover firsthand. Eye contact hurts and accidental ingestion? Calling poison control and a trip to the hospital often follows.
In the environment, spills or poorly managed storage make for groundwater problems. Strict guidelines keep these events rare, but they start with each person understanding where things can go wrong and setting up better habits.
Storing this chemical never feels dramatic, but small mistakes add up. Sealed, airtight containers block moisture and air that could degrade the substance or spur accidental reactions. Even a tiny leak leads to slow spills, invisible dust, and the kind of mess that never vaccums up completely.
Choosing metal or glass over plastic helps, since strong, inert materials resist corrosion and provide a reliable barrier. Label every container with big, clear writing—no abbreviations, no guesswork.
Shelving matters too. Place jars of 2-Methylanthraquinone on a low, stable shelf, well away from acids, alkalis, and anything likely to react. Squeeze bottles and reused containers may seem convenient on a busy day, but swapping them for purpose-built options pays off. Out of direct sunlight, in a cool and dry spot, keeps degradation at bay.
Lab coats, gloves, and goggles aren’t just props. They shield bare skin and eyes from splashes and offer a line of defense in case bottles tip or powders get airborne when scooping material onto a scale. Fume hoods draw away dust before it lingers in the air—no one enjoys a throat full of chemical particles.
People sometimes skip steps in a rush, but keeping a spill kit close pays off. Sand, absorbent pads, or dedicated chemical neutralizers stop leaks in their tracks. Well-kept eyewash stations and emergency showers are more than regulatory boxes to check: quick action after a splash stops incidents from turning worse.
Teaching new lab members or production staff about respectful handling becomes an ongoing habit. Share stories of near-misses or lessons learned—these stick longer than any printed safety sheet. Keep material safety data sheets nearby, but also walk through procedures step-by-step during training.
Taking a little more time with each transfer or weigh-out prevents headaches later. Open bottles slowly, scoop gently, and seal things up immediately after use. Label waste before disposal and store hazardous leftovers apart from other production areas, far from busy walkways.
Many workspaces review which chemicals are truly necessary and swap out those with higher hazards if safer options work as well. Regular safety reviews and real conversations about incidents—even the ones nobody wants to admit—keep risks in check. From my own days cleaning up after a spill, there isn’t a shortcut worth taking. The right habits save time, money, and peace of mind.
2-Methylanthraquinone has become essential for several key industries, especially for those in the paper and pulp sector, and for anyone working in organic synthesis or dye manufacture. I’ve seen this compound used most by clients involved with pulping operations, where it improves yield and cuts cooking times. Its higher purity forms also go to labs or companies developing dyes, pigments, or specialty chemicals.
Most buyers look to established chemical suppliers for bulk purchases. Sigma-Aldrich, TCI and Alfa Aesar offer reliable service, making them common choices for labs and manufacturing plants. For larger volumes, I’ve talked with purchasing managers who prefer regional distributors or direct contact with international firms in China or India. These firms often quote competitive prices for pallet or drum quantities and handle export paperwork efficiently.
Large-scale buyers sometimes check Alibaba or Made-in-China.com, which list manufacturers and trading companies offering 2-Methylanthraquinone by the metric ton. Those websites give access to suppliers who specialize in industrial-grade batches, though businesses need to invest time vetting suppliers’ quality assurances and shipment schedules. Relationships with these suppliers can open doors to better pricing or tailored specifications over repeat orders.
Before placing a bulk order, checking certifications and understanding the product’s quality is key. Buyers need a certificate of analysis, batch sample, or third-party lab report. Professional suppliers rarely hesitate to share these. I’ve learned to watch for companies with credibility in safety and handling, as chemical mishaps in storage or transport hit the bottom line fast.
Regulations add another layer. In the United States, 2-Methylanthraquinone doesn’t show up on highly restricted lists, but large deliveries often raise red flags for shipping and customs brokers unfamiliar with harmless paperwork. After helping a small dye maker in California import their first drum, I suggest getting regulatory advice at the start, saving headaches down the line.
Startups and small firms get hit by high minimum order quantities and tight cash flow. Plenty of newcomers try to split orders with other buyers, but cooperative purchasing only succeeds with clear agreements. Some distributors offer sample or trial packs at a slightly higher price per kilogram, which helps teams running R&D projects before scaling up.
Existing relationships with distributors speed up lead times. Each time I’ve worked with clients upgrading their purchasing operations, I push them to find a sales rep who takes their needs seriously. Fast responses, transparent lead time estimates, and honesty about supply chain hiccups build trust and keep operations running smoothly.
Sustainable sourcing can offer a business edge, especially as buyers and regulatory agencies focus more on environmental impact. While few suppliers focus on “green” production of this compound, some do highlight waste minimization or compliance with ISO standards. Smart buyers ask questions about manufacturing methods and supplier audits before locking in a multi-year deal.
Plenty of options exist for securing 2-Methylanthraquinone in bulk, from local distributors to direct sourcing abroad. Buyers who build strong partnerships, demand transparency, and keep compliance top of mind put themselves in the best position to obtain quality material, on time, at a reasonable price.
| Names | |
| Preferred IUPAC name | 9-Methylanthracene-9,10-dione |
| Pronunciation | /tuː ˌmɛθɪlˌænθrəˈkwɪnoʊn/ |
| Identifiers | |
| CAS Number | 84-54-8 |
| 3D model (JSmol) | `3d:JSmol|C1=CC2=C(C=C1C3=CC=CC=C3C(=O)C2=O)C` |
| Beilstein Reference | 1290627 |
| ChEBI | CHEBI:28638 |
| ChEMBL | CHEMBL140720 |
| ChemSpider | 14286 |
| DrugBank | DB13254 |
| ECHA InfoCard | 100.014.280 |
| EC Number | 204-626-7 |
| Gmelin Reference | 74069 |
| KEGG | C16535 |
| MeSH | D018101 |
| PubChem CID | 7096 |
| RTECS number | CB8200000 |
| UNII | 3X6188J2K6 |
| UN number | UN2564 |
| CompTox Dashboard (EPA) | DTXSID7034308 |
| Properties | |
| Chemical formula | C15H10O2 |
| Molar mass | 254.27 g/mol |
| Appearance | Yellow needles or yellow crystalline powder |
| Odor | Odorless |
| Density | 1.318 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 3.7 |
| Vapor pressure | 0.0000727 mmHg at 25°C |
| Acidity (pKa) | 13.0 |
| Basicity (pKb) | 10.61 |
| Magnetic susceptibility (χ) | -77.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.657 |
| Viscosity | 2.25 mPa·s (at 130 °C) |
| Dipole moment | 2.77 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 331.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -82.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5725 kJ/mol |
| Pharmacology | |
| ATC code | D05BA02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | InChI=1S/C15H10O2/c1-10-6-7-12-13(8-10)15(17)11-5-3-2-4-9(11)14(12)16/h2-8H,1H3 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 205°C |
| Autoignition temperature | 515 °C |
| Lethal dose or concentration | LD50 (oral, rat): 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| NIOSH | WF4375000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/m³ |