2-Chlorophenol first showed up in chemical texts and patent files in the early 20th century, rising with the broader phenol family during a boom in antiseptics and dye intermediates. Early chemists worked with crude processes, often breathing in harsh vapors and wearing heavy wool coats as their only safety gear. As industry scaled up, better purification and process controls replaced unpredictable batch runs. By the 1950s, chemical plants shipped 2-chlorophenol across borders, listed in trade directories and regulatory bulletins. The product moved from lab benchtops in glass stoppered bottles to tankers and bulk drums rolling toward paper mills and resin factories.
2-Chlorophenol carries the chemical formula C6H5ClO, packing both chlorinated and hydroxyl groups on a benzene ring. You catch a medicinal, almost musty odor wafting from pure crystals. Over decades, manufacturers carved out a space for it in wood preservation, biocides, solvents, plastics, and pharmaceutical intermediates. Companies list it under names like o-chlorophenol, ortho-chlorophenol, and 1-hydroxy-2-chlorobenzene. You spot these names on safety sheets, supply catalogs, and customs declarations. It’s not glamorous, but it delivers the reactivity needed for specialty syntheses and niche industrial jobs.
You unpack 2-chlorophenol and see colorless to slightly amber crystals, sometimes turning tan from exposure. The melting point usually hovers near 8.7°C, so it can show up as an oily solid or a low-melting wedge, depending on warehouse drafts. Its boiling point lands just above 175°C, putting it well within the ordinary range for halogenated phenols. Not especially soluble in cold water, but it mixes easily with most organic solvents — diethyl ether, benzene, ethanol, and the like. Acidity lines up with other phenols, placing its pKa around 8.5, a notch more acidic than pure phenol thanks to the electron-withdrawing pull from the chlorine. Laymen rarely notice such subtlety, but researchers fine-tune extraction and synthesis with these numbers.
Bulk containers carry warning labels — the UN number 2021, proper hazard pictograms, and accurate concentration information. Catalogs offer purity grades, commonly at 98% or higher, and specify limits on impurities like 4-chlorophenol or residual solvents. Packaging relies on polyethylene or metal cans sealed tight against air and moisture. Labels spell out batch numbers, manufacturing dates, and lot traceability. This helps chemical buyers meet national and international transport standards and trace every shipment back to plant origin. Any slip-up can prompt costly recalls and investigations, as even small contaminants affect downstream reactions or environmental compliance.
Most large-scale processes for 2-chlorophenol start with phenol, using electrophilic aromatic substitution. Chlorine gas or sodium hypochlorite attacks the ortho position, guided by the activating effect of the hydroxyl group. Yields vary by catalyst, temperature, and time, with iron(III) chloride or copper chloride used to finesse the process. Small-scale preps sometimes lean on monochlorination of molten phenol, though over-chlorination threatens purity. Waste streams demand careful neutralization and scrubbing to manage corrosive byproducts and volatile organics. This step matters: many communities still recall plant leaks or improper dumping that tainted water supplies in the postwar years, fueling tough environmental controls.
The chlorine atom on 2-chlorophenol opens doors for substitution reactions — think of nucleophilic aromatic substitutions turning it into aminophenols for hair dyes, or aldehyde condensation for antiseptic resins. Researchers regularly tweak reaction conditions to make pharmaceuticals, plasticizers, dyes, and agrochemical intermediates. The phenolic OH gets involved too, forming esters and ethers at the hands of skilled organic chemists. In my experience, every college teaching lab with a focus on aromatic chemistry eventually tries to swap out that chlorine, teaching students about activating and deactivating groups on the aromatic ring. Still, protecting groups and reaction order matter — careless choices cost both money and time.
You find 2-chlorophenol in the market under half a dozen trade names: o-chlorophenol, ortho-chlorophenol, Aseptoform, and at times, simply “chlorophenol” in less regulated counties. Each name crops up with regional spelling twists and supplier labels. The molecule gains new code numbers and short product handles as it passes between inventory software, shipping clerks, and customs warehouses. These synonyms help clear up orders with broad-spectrum buyers while sometimes confusing new students or less experienced handlers. Regulatory sheets, customs documents, and global sales agreements often stuff several of these names side by side.
2-Chlorophenol bears a hazard classification for toxic and corrosive substances. Inhalation burns the lungs and mucous membranes; skin exposure tingles or blisters without proper gloves. Long-term exposures risk liver and kidney damage, echoing tragic cases from poorly ventilated pulp mills and pesticide labs back in the 1970s. Strict ventilation, eye protection, and chemical-resistant gloves stand as non-negotiable in modern facilities. Storage demands cool, dry conditions and emergency eyewash stations within quick reach. Emergency plans spell out spill management, safe disposal, and first aid, not just as box-ticking exercises, but because real people face the sharp reality of an accident. MSDS sheets detail symptoms, exposure limits, and proper personal protective equipment with no room for shortcuts. Regular safety drills and open conversations save lives, ensuring no worker or student ends up as another cautionary tale.
Industries use 2-chlorophenol in wood preservation, biocides, industrial solvents, and intermediate synthesis. Paper and pulp mills treat logs with it to block fungal rot. Biocide manufacturers blend it for antimicrobial sprays and hospital disinfectants where broad-spectrum activity helps control outbreaks. In pharmaceuticals, it acts as a stepping stone to active ingredients and dyes. Other sectors tap into its reactivity for custom polymers, adhesives, and advanced coatings. My own experience working with industrial chemists revealed a constant search for alternatives as health and environmental rules grow tighter, but the unique molecular structure of 2-chlorophenol keeps it in rotation for specialized tasks that newer chemicals struggle to match.
R&D teams, both private and academic, run a steady stream of studies focused on greener synthesis methods, lower toxicity analogues, and targeted applications. Green chemistry efforts seek to swap in milder chlorinating agents or develop catalytic systems that slash waste and emissions. Some groups adapt continuous-flow reactors to make the process safer with less operator exposure. Toxicologists map out the mechanism of cellular injury after low-dose exposure, searching for ways to block damage. Patents pile up for derivatives with promising antimicrobial traits and better safety profiles. Interdisciplinary collaborations link organic chemists, engineers, and medical researchers, chasing molecules that do the job with fewer hazards at every step from bench to disposal.
Toxicologists flagged 2-chlorophenol as hazardous decades ago, with animal studies reporting liver and kidney injury, respiratory irritation, and developmental effects at high exposures. The U.S. EPA and European regulators keep it on priority monitoring lists, setting occupational exposure limits and classifying it as harmful to aquatic life. Its ability to persist in water and soil builds the case for continued monitoring in post-industrial areas. Poison control centers and environmental lawyers recall cases of groundwater contamination and accidental worker poisonings, underscoring the need for tough engineering controls, secure storage, and strict training. The environmental persistence of halogenated phenols lingers in public memory, driving calls for ongoing studies into safer substitutes and improved remediation techniques.
As green chemistry advances, research trends aim to minimize production hazards, trim emissions, and cut waste. Regulatory frameworks tighten every year, pushing manufacturers toward closed systems and life-cycle management. Academic teams hunt for substitutes that balance efficacy, cost, and environmental impact, while policymakers lean on updated risk assessments to protect workers and consumers. The industrial roots of 2-chlorophenol run deep, and for all the talk of alternatives, its role in pharmaceutical intermediates, specialty biocides, and preservative blends keeps it in steady demand. Progress will likely mean different synthesis routes, stricter documentation, and smarter handling — not total phase-out, at least until every downstream product finds a safer path. Responsible manufacturers and labs already take cues from new best-practices, automating hazardous steps and investing in personal protective technologies so that future generations never relive mistakes from the past.
2-Chlorophenol stands out in the world of chemicals, with a role that reaches far beyond the lab. This compound has built its reputation as a workhorse for making other chemicals. Many folks who work in agriculture or handle industrial materials have used products made with 2-Chlorophenol, maybe without realizing it. Factories rely on it to kickstart larger reactions. Experience in the chemical industry highlights its use in the making of pesticides, herbicides, and some dyes. Anyone who has watched the process from raw material to finished product knows how much one small ingredient can influence the chain.
Industrial processes often depend on simple building blocks to create more complex substances. 2-Chlorophenol is often one of those key building blocks. Chemical plants use it to produce other phenolic compounds, which eventually end up in disinfectants, antiseptics, and even materials used in manufacturing plastics or rubber. Its antiseptic qualities lead to its inclusion in certain sanitizing agents.
Products designed to fight fungus and bacteria tap into these properties. Experience working in a manufacturing environment brings a certain respect for these multipurpose materials. Staff must always stay alert, since handling 2-Chlorophenol involves extra precautions due to its toxic nature. Proper training and effective safety protocols can prevent the headaches and health issues associated with accidents.
Chemicals like 2-Chlorophenol come with a real need for responsibility. The Centers for Disease Control and the Environmental Protection Agency both stress the importance of not taking shortcuts during handling or disposal. In the past, people dumped waste without much thought for the environment. We have learned the hard way. Cases linked to contamination show up now and then, especially near old manufacturing sites or where regulation ran thin. Serious health problems such as liver and kidney damage or skin burns happened when guidelines were ignored.
Taking these risks seriously means insisting on better storage and focusing on employee education. Years spent on-site drive home the lesson that nothing beats awareness. Companies must limit exposure, use sealed systems, and treat waste before it goes anywhere near a water supply. Communities living near plants have a right to clear communication about any risks.
Relying less on substances with high toxicity remains a challenge in chemical manufacturing. Green chemistry has opened new doors, with researchers constantly hunting for alternatives. Some new catalysts and processes produce similar results using safer raw materials. Even if removing 2-Chlorophenol from every process seems out of reach right now, pushing for change within the industry matters. In some countries, tighter environmental rules forced companies to innovate, cutting use and releasing cleaner products.
Informed decisions at every stage—from procurement to disposal—protect both workers and communities. A future with cleaner manufacturing requires everyone to stick to strong practices, demand better substitutes, and never lose sight of the human cost tied to each decision.
Anyone who has spent time around a chemical lab knows 2-Chlorophenol by its sharp smell. It packs a punch long before you open a label. Just a whiff signals danger. For those new to it, this compound can irritate the skin and burn the eyes. Breathing vapors for a few minutes makes your throat and lungs scream. Getting careless with storage or cleanup leaves you wide open to accidents. Even after decades working with hazardous materials, that uneasy respect for chemicals like this never fades.
Latex gloves alone won’t hold up against 2-Chlorophenol. It soaks through, and skin rash comes next. Chemical-resistant gloves—nitrile or neoprene—outlast the first splash and keep the burn at bay. Safety goggles are a must. Regular eyewear isn’t enough if vapors start swirling. Sometimes, those same vapors slip out of a beaker and sting your eyes before you notice. An organic vapor respirator adds peace of mind in small, stuffy rooms. I remember a day when someone splashed a few drops on their lab coat; the stinging smell lingered for hours, and that coat hit the disposal bin right away.
Strong exhaust fans and fume hoods are lifesavers. Any task with liquid handling belongs under a hood. Closing a lid fast stops vapors from crawling across the room. Locked storage in a cool, dry place keeps accidents down. Diluted acids or open windows don’t cut it. Corrosive-resistant cabinets guard against leaks—especially if someone's distracted and forgets to double-check a cap. I’ve seen plastic containers warp over time, leaving a pool at the bottom of a shelf. Polyethylene and amber glass bottles always hold up better in real labs.
A sudden spill can turn a routine task into a day-long cleanup. Paper towels make things worse—the compound simply seeps through. Absorbent pads designed for hazardous chemicals pull the liquid in quick. Wearing gloves, scooping up broken glass, and tossing all materials into hazardous waste bags prevents any after-hours nastiness. Washing any splashed skin right away with cold water works better than reaching for special cleansers. Ample fresh air after a spill clears out those lingering fumes faster than air conditioning.
Every lab or industrial site benefits from honest, upfront training. No one remembers every section of a protocol binder, but hands-on drills pay dividends. After years of practice, the speed of group response stands out during emergencies. Eye wash stations and safety showers near work areas turn a serious exposure into a minor inconvenience. Teams with clearly assigned roles handle a spill in seconds. I’ve watched new staff freeze during their first chemical alarm until a veteran calmly guided them through cleanup, showing the value of practice over paperwork.
Replacing open beakers with sealed vessels cuts exposure in half. Using pre-measured, sealed ampoules or single-use kits means fewer chances for drips or spills. Automation and remote handling tools let people step back when possible. Industries making these changes see fewer incidents. Workers spend less time worried about hidden leaks or surprise reactions, and more time focused on real work. Technology advances, but the basics—respect the chemical, work with safe gear, and act fast when accidents happen—keep everyone safer.
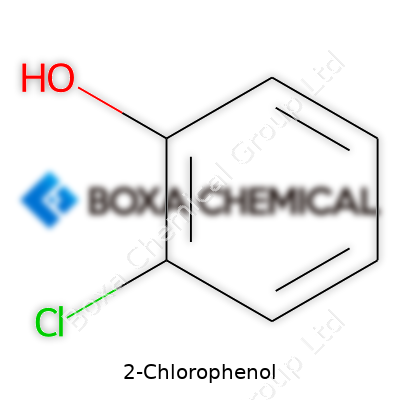
Chemists call it 2-Chlorophenol, but its formula is much simpler: C6H5ClO. It comes from the phenol family—aromatic rings with an -OH group, and it has a chlorine atom swapped onto the second carbon next to the hydroxyl. The benzene ring stays solid at the center of this structure, holding one hydroxyl group at the number one spot and chlorine right next to it at number two.
The structure adds more than just a code to a name. Lay out the atoms, and you see a hexagon (the benzene core), showing alternating double bonds, an -OH sticking to one carbon, and a Cl clinging to its neighbor. This layout changes how the molecule reacts—chlorine and hydroxyl bring their own quirks, and their spot in the ring matters.
I learned about 2-Chlorophenol in organic lab around the same time we covered household cleaners. Both safety and usefulness start from the fact that having chlorine near the hydroxyl group makes it much more reactive. In practice, this means a stronger antiseptic punch, and labs use it for making pesticides, dyes, and even drugs. The way those atoms connect is what makes it so interesting for synthesis—reacting more easily than plain phenol.
Reactiveness comes at a price. 2-Chlorophenol can irritate your skin and lungs. It has a strong, unmistakable odor that means business. Environmental concerns are real—the chlorinated part resists breaking down out in nature, and that’s a lesson we keep learning in cleanup sites and water testing labs. I’ve seen tests for 2-Chlorophenol in contaminated soil samples, and the challenge always comes down to how persistence ties back to its shape. Its chemical stubbornness is exactly what makes it useful—and makes cleanup tough.
In manufacturing, even a small change from one position on the benzene ring to another creates a new chemical. 2-Chlorophenol’s ortho position (chlorine at the second spot) changes reactivity compared to, let’s say, the para version (chlorine at the fourth spot). This isn’t just academic—companies choose one over the other based on the outcome they want. While I worked with waste chemical samples, I learned that the ortho version breaks down slower. That meant longer monitoring windows and more effort to protect water nearby.
Handling this compound, gloves and fume hoods are non-negotiable. Having a spill response on standby is standard because its volatility turns a drop into a big mess. Safety data sheets don’t just check a box—they reflect real hazards tied to the structure of the chemical.
Regulators keep 2-Chlorophenol in their sights, right up there with other chlorinated phenols. The EPA calls it a priority pollutant. Solutions start with policies that place limits on industrial release and encourage greener alternatives. Stronger rules on discharge help keep local water safer. At the same time, newer chemistry keeps pushing for solutions that replace persistent chemicals with designs that break down easier.
Education helps too. Teaching students to recognize chlorinated phenols’ structure connects textbook chemistry to what they might find on the job. Respect for the molecule’s risks and history keeps labs, factories, and the environment a little bit safer.
2-Chlorophenol may sound like just another chemical among thousands out there, but people who work around it know its hazards. With a sharp odor that hits your nose even at low concentrations, this compound demands respect. Its health risks stack up quickly, from skin burns to long-term organ damage. Stories of accidental splashes and poor ventilation remind us how dangerous things get if storage precautions fall short. My own early years in the lab taught me this: never assume a chemical won’t escape a bottle just because the cap seems tight.
Many accidents start with poor storage choices. Storing 2-Chlorophenol involves picking containers made from materials that stand up to potent chemicals. Polyethylene or glass with Teflon-lined caps hold up better than standard plastics. In one lab I worked at, a technician grabbed an unknown bottle off a shelf and the cap, half-dissolved, released a nasty puff of vapor. Everyone learned two things that day: always double-check containers, and keep incompatible chemicals away. This compound reacts with strong oxidizers, acids, and bases, so it belongs nowhere near them.
People often overlook the effect of heat or sunlight, but these cause the chemical to break down or evaporate more quickly. A dark, well-ventilated cabinet works best. I have seen budget facilities try to cut corners with basic closets, only to deal with sick workers and ruined supplies. Fumes can collect quickly, so local exhaust ventilation or a dedicated chemical fume hood saves headaches—sometimes in the most literal sense.
Every bottle ought to carry a clear, readable label. In my own time supervising a small academic storeroom, even sloppy handwriting once delayed an emergency response when someone knocked over a container. Time lost while guessing what you’re cleaning up only increases the chance of injury. Spill kits designed for chlorinated organics should sit nearby, stocked with absorbent materials that handle volatile liquids. A good habit: always check the kit before you store fresh bottles; sometimes supplies run low without anyone noticing.
Simple routines save lives. Gloves, goggles, and lab coats stop direct skin contact and eye injury, but respirators may become necessary if there’s ever a spill or leak in a tight space. I always insisted on weekly checks—looking for any sign of a bottle degrading, cracks, or crust around the cap. Routine inspections catch leaks before they become a problem. Training new staff every year, no matter how boring it seems, prevents shortcuts and complacency.
Storage rules may sound bureaucratic, but real-world experience proves they’re written in the aftermath of accidents. Every seasoned chemist or technician has a story to tell. As someone who has cleaned spills and bandaged burns, I recommend careful handling, smart storage choices, regular checks, and loud reminders about the risks. Safe storage is not just about the law—it keeps you, your coworkers, and the building safe.
Few people walk into work thinking they’ll run into 2-Chlorophenol, but plenty of industries use this chemical each day, especially in making pesticides, dyes, and antiseptics. Anyone working in these environments faces real health concerns—concerns backed up by incidents and research. I’ve seen colleagues work through strange smells and burning eyes in similar factory settings. It doesn’t take much for a seemingly small problem to grow into a health crisis.
Skin splashes and inhalation spark red flags right away. 2-Chlorophenol can burn through gloves and irritate skin, often leading to redness, blisters, or persistent itching. Without solid protective gear, exposure means cracked skin that can open the door to infection, something no hand-washing routine can quite fix. Quick exposure to the vapor causes sharp coughing and breathing trouble. Even small, fleeting amounts can set off severe headaches and dizziness.
Workers who keep getting exposed to low levels run a risk that’s easy to miss until it’s too late. Over time, the liver and kidneys get hit the hardest. The chemical builds up quietly, straining these organs until small aches become chronic problems. Evidence from studies— including one from the European Chemicals Agency— ties ongoing exposure to long-term effects like liver dysfunction and potential kidney impairment. This isn’t theory; it’s happened in workplaces with inadequate controls.
I’ve talked to people who just wanted to finish a shift and noticed numbness in their hands or changes in how they think after spending weeks around chlorinated chemicals. Chronic toxic effects like these grow slowly, so folks brush them off for months, sometimes longer.
It’s not just industrial workers. Poor ventilation drags this problem home. Vapors linger in the air, getting trapped indoors. Family members end up breathing what’s left behind on work clothes or shoes without realizing it. More than once, someone has noticed strange chemical odors at home after handling work gear, and that air brings its own risks, including respiratory irritation and long-term lung issues.
The health community points toward well-proven strategies. Wearing proper gloves and face masks sharply lowers the risk during handling. Regular air quality checks catch slow leaks or accidental spills. Basic skin hygiene—true hand-washing and changes of clothing—makes a real difference. Legally, regulations back up these steps. OSHA and similar agencies keep records and set strict exposure limits that employers must follow, using science to justify them. If companies fall short, cases of recorded poisonings become the proof needed for penalties.
Better education around hazardous work ensures people understand what dangers look and feel like, not just what the regulations say. Real stories make the need for action stick. Everyone should know: safety precautions are rooted in real consequences, not formality. Any shortcut puts health directly at risk, not just for individuals but their families and communities as well.
| Names | |
| Preferred IUPAC name | 2-Chlorophenol |
| Other names |
o-Chlorophenol ortho-Chlorophenol 1-Chloro-2-hydroxybenzene 2-Hydroxychlorobenzene |
| Pronunciation | /tuː ˌklɔːrəˈfiːnɒl/ |
| Identifiers | |
| CAS Number | 95-57-8 |
| Beilstein Reference | 1209223 |
| ChEBI | CHEBI:16299 |
| ChEMBL | CHEMBL14085 |
| ChemSpider | 6612 |
| DrugBank | DB11383 |
| ECHA InfoCard | EC Number: 200-293-7 |
| EC Number | 2.1.1.1 |
| Gmelin Reference | Gmelin Reference: 8280 |
| KEGG | C01356 |
| MeSH | D002766 |
| PubChem CID | 1621 |
| RTECS number | GR7875000 |
| UNII | 5C91DE8447 |
| UN number | UN2019 |
| CompTox Dashboard (EPA) | DTXSID2020718 |
| Properties | |
| Chemical formula | C6H5ClO |
| Molar mass | 128.56 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | aromatic; penetrating |
| Density | 1.265 g/mL at 25 °C(lit.) |
| Solubility in water | 18 g/L (20 °C) |
| log P | 2.15 |
| Vapor pressure | 0.479 mmHg (25°C) |
| Acidity (pKa) | 8.5 |
| Basicity (pKb) | 14.34 |
| Magnetic susceptibility (χ) | -61.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.553 |
| Viscosity | 2.41 mPa·s (25 °C) |
| Dipole moment | 1.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 111.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -62.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3790 kJ/mol |
| Pharmacology | |
| ATC code | D08AE04 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation, toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H302, H312, H315, H318, H332, H401 |
| Precautionary statements | Precautionary statements of 2-Chlorophenol: "P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P311, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0-Acidity |
| Flash point | Flash point: 81 °C (178 °F; 354 K) |
| Autoignition temperature | 605 °C |
| Explosive limits | 2.7% - 12.7% |
| Lethal dose or concentration | LD50 oral rat 530 mg/kg |
| LD50 (median dose) | LD50 (median dose): 530 mg/kg (oral, rat) |
| NIOSH | C642 |
| PEL (Permissible) | 5 ppm |
| REL (Recommended) | 5 mg/L |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Phenol 2-Bromophenol 2-Iodophenol 2-Fluorophenol 3-Chlorophenol 4-Chlorophenol Aniline |