In the late 1800s, chemists searching for new dyes and pharmaceuticals took a closer look at 2-aminophenol. Wilhelm Körner first identified it in the 1870s, drawn by its unique mix of amino and hydroxyl groups on the benzene ring. Researchers in dye chemistry saw huge potential for crafting vibrant colors. Early manufacturers produced 2-aminophenol through reduction of nitrophenols, paving the way for broader use across European and American laboratories. By the 20th century, this compound appeared as a staple in organic synthesis toolkits. Historical curiosity and industry demand pushed its development, and today’s applications still echo those early insights.
2-Aminophenol forms as a crystalline solid, often pale pink or off-white, and gives off a faint odor at room temperature. Chemists and manufacturers handle it as a valuable intermediate, especially where selective reactions are needed. Its structure, with an amine and hydroxyl group directly attached to a single phenyl ring, gives it substantial reactivity and makes it suitable for creating a range of compounds, from photographic developers to fine chemicals and specialty polymers. Suppliers standardize it for laboratory, pharmaceutical, and industrial use, meeting the needs of different sectors with consistent quality controls.
As a solid below 174°C, 2-aminophenol melts fairly easily compared to other substituted benzenes. It dissolves well in hot water and polar organic solvents, which works to its advantage in chemical processing. The molecule (C6H7NO) weighs just 109.13 g/mol, so it disperses quickly in reaction mixtures. Its dual functionality allows the molecule to act both as an acid (through the hydroxyl group) and a base (through the amine). In practice, that flexibility makes it a favorite for complex synthesis. Chemists observe that exposure to air and light gradually darkens its color, highlighting the need for proper storage.
Producers routinely label 2-aminophenol under CAS number 95-55-6. Purity often exceeds 99%, as low levels of impurities impact performance in sensitive applications. Product sheets specify melting points (170-174°C), solubility data, and spectral characteristics (notably NMR and IR peaks). Packaging includes hazard symbols required under US OSHA and EU REACH rules, along with handling instructions reminding users of its irritant potential. Industrial buyers can request certificates of analysis verifying batch consistency, and safety data sheets contain full regulatory and storage information.
Modern processes synthesize 2-aminophenol by reducing 2-nitrophenol, using iron filings and hydrochloric acid or catalytic hydrogenation. Labs may also run hydrolysis of 2-aminophenyl ethers in the presence of acid. Yields run high when oxygen is excluded, and industry setups recycle by-products for waste minimization. These pathways favor accessible reagents and scalable protocols, matching the speed and efficiency required for high-volume production. Many researchers recall learning the reduction process as a core step in their organic lab rotations, cementing its reputation as a reliable experiment.
The amine and hydroxyl moieties open a wide field of chemical modification. Condensation with aldehydes, etherification, diazotization, and coupling to create azo dyes all get their start using this molecule. For example, reacting 2-aminophenol with benzaldehyde can form stable Schiff bases, while direct acylation yields N-acyl-2-aminophenols, important in medicinal chemistry. Oxidation produces o-quinone imines, which have biological interests and find use in research labs. Chemists develop new ligands and chelating agents, building off the molecule’s bidentate character, and recent patents focus on creating new catalysts based on its skeleton.
In catalogs and literature, 2-aminophenol appears as ortho-aminophenol, o-aminophenol, 2-hydroxyaniline, or 2-hydroxybenzenamine. Bulk suppliers and scientific distributors, such as Sigma-Aldrich and Thermo Fisher, group it under these synonyms to streamline ordering. Healthcare and regulatory bodies also reference it in toxicology and chemical registrations under these alternate names. Researchers must stay alert to these variations, as some countries still use region-specific naming conventions.
Handling 2-aminophenol safely calls for gloves, goggles, and effective ventilation. The substance irritates eyes, skin, and respiratory tracts, so companies enforce strict exposure limits. Storage takes place in tightly sealed containers, away from oxidizers and sunlight, to reduce degradation and color change. The US Environmental Protection Agency and European regulators assign it classified irritant status, and large-scale operators adopt continuous air monitoring and spill containment. On-site training stresses immediate washing if contact occurs. Local fire and environmental codes dictate response plans in the case of large spills or accidental releases, establishing robust standards industry-wide.
Photographers associate 2-aminophenol with traditional black-and-white film developers like Rodinal, where its reducing power creates bright, crisp images. Dye manufacturers use it for crafting indophenol dyes, needed in hair coloring, textiles, and printing inks. Pharmaceutical chemists see 2-aminophenol as a precursor for certain anesthetics and anti-tuberculosis drugs. On a larger scale, it finds roles in polymer synthesis, water treatment, and as a corrosion inhibitor. Laboratory researchers deploy it to make specialized ligands and sensors. Each field demands different purities and packaging, illustrating how one compound serves a surprising range of technical communities.
Scientific teams continue to probe the potential of 2-aminophenol for new materials and therapeutic agents. Chemical engineers tweak its reactivity to create high-performance polymers, while pharmaceutical labs investigate derivatives that can serve as enzyme inhibitors or diagnostic tools. Recent journal articles discuss using the compound in sustainable oxidation processes, hinting at lower-waste production of fine chemicals. Multinational collaborations tap green chemistry principles, shifting away from heavy-metal catalysts to more benign reaction conditions using 2-aminophenol templates. In my own graduate work, experiments with substituted aminophenol derivatives revealed new patterns in molecular recognition, fueling interest in this compact but versatile molecule.
Toxicologists track both acute and chronic effects of 2-aminophenol exposure. At higher concentrations, it causes skin and eye irritation, and inhalation can irritate airways. Repeated contact over time raises concerns about systemic toxicity. Animal studies have shown some impacts on kidney and liver function at elevated doses, which influences workplace safety regulations. Regulatory agencies incorporate these findings into chemical hazard assessments, while researchers seek ways to lower exposure in industrial settings. In controlled laboratory environments, fume hoods and personal protective equipment keep risks low, but mishandling or spills in manufacturing plants remain a safety focus.
Looking ahead, 2-aminophenol’s legacy offers plenty of reasons for chemists to keep exploring fresh uses. Green chemistry efforts point toward more efficient, lower-waste synthetic methods. The pharmaceutical sector tracks its derivatives as potential leads for targeting neurological disorders or infectious diseases. Environmental engineers view it as a feedstock for new biodegradable polymers. Nanotechnology researchers experiment with self-assembled structures based on its shape. As scientific knowledge expands, this once-obscure compound continues to drive inventive chemistry and cross-disciplinary innovation, reminding us of the value hidden in small molecules from the dawn of modern science.
2-Aminophenol often hides behind the curtain in everyday products and lab work, but its influence runs deep. In the lab, this compound pops up in the recipes for creating dyes—those colors in textiles, plastics, even in some inks that stick to favorite paperbacks. Over time, chemists figured out the special bond between 2-aminophenol and color. Its structure, with both an amino group and a hydroxyl group attached to a benzene ring, allows it to work as a versatile building block. This structure helps it latch onto other molecules to form larger, more complex compounds, including popular colorants like azo dyes. The root of many vibrant reds, blues, and violets in clothing and plastics can be traced back to this chemical, which has proven stable and reliable in delivering color that lasts.
Headaches, fevers, and pain get treated with medicines that rely on solid, consistent chemistry. 2-Aminophenol acts as a starting point for several pharmaceutical compounds. Acetaminophen, called paracetamol in most places, relies on this molecule during production. Memories of treating a fever or reaching for pain relief highlight the real impact of this chemical: it is part of the backbone of something trusted worldwide. Drug manufacturing demands strict quality and safety controls, so chemists favor compounds like 2-aminophenol that demonstrate predictable reactions.
Older photography, especially in black-and-white film, trusted chemistry to freeze a fleeting scene. Developers that turned negatives into lasting prints often depended on 2-aminophenol because of its reducing properties. This compound helped change exposed silver halides into metallic silver, revealing clear images. Hobbyists, professionals, and historians owe a debt to this compound for its role in giving permanence to everyday memories before digital took over. Even today, some photo enthusiasts pick up bottles with this ingredient for traditional home darkrooms, preserving analog skills and art.
Dyes and pharmaceuticals stand out, but 2-aminophenol contributes to several niche specialties. It goes into rubber chemicals that keep tires tough, making sure cars hold up against the wear of daily driving. It also serves as an intermediate when making certain agricultural chemicals—helping farmers tackle threats on the field and boost harvests. For those in labs, 2-aminophenol becomes a reagent for spotting other chemicals or measuring substances. Its flexibility makes it a favorite among chemists who need results they can trust.
Handling chemicals always brings safety and responsibility into the conversation. 2-Aminophenol has shown some concerns for skin irritation and needs careful handling, especially in workplaces where exposure could be routine. Proper storage and disposal matter, especially since accidental release can affect water and soil. By sticking with protocols like using protective gear and proper ventilation, labs keep their people and the environment safer.
The world faces more scrutiny today about how chemicals influence health and the environment. Where possible, green chemistry principles shift focus toward safer handling, biodegradable alternatives, and closed-loop manufacturing. Academic centers and the chemical industry continue to develop cleaner methods for producing and recycling compounds like 2-aminophenol, hoping to leave fewer marks on the planet while keeping the benefits people have come to rely on.
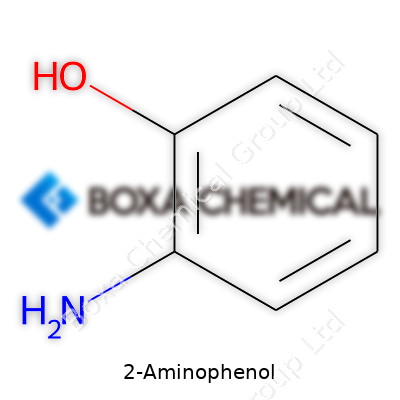
2-Aminophenol sticks out in the world of organic chemistry for its dual-function groups sitting on a simple benzene ring. Its formula, C6H7NO, shows this molecule has six carbons, seven hydrogens, one nitrogen, and one oxygen. This formula tells you there's a benzene backbone, tweaked by the addition of an amino group and a hydroxyl group. The numbers might seem simple, but arrangement always runs the show in organic chemistry.
On paper, 2-aminophenol features an amino group (-NH2) and a hydroxyl group (-OH) bonded directly to the benzene ring. The “2-” in the name, or “ortho” placement, means these two functional groups stand side by side — both attached to adjacent carbons on the ring. Chemistry textbooks use this layout all the time, showing the ring as a hexagon with the two groups sitting next to each other, responsible for shaping the molecule’s personality. Each group brings its own reactivity, sometimes acting as a donor, other times as a recipient in reactions involving electrons.
Everyone who’s spilled some on a glove remembers the distinct scent and a hint of a stain. The deep brownish or off-white color, the feel of its easy solubility in hot water — these aren’t just quirks. They come from how that hydroxyl and amino group shape the electronic structure, making 2-aminophenol interact differently than a simple phenol or straight aniline.
Anyone who spends time in a chemistry lab quickly gets a sense of the practical side. The arrangement of those two groups turns this molecule into a useful intermediate for building dyes, medicines, and complex polymers. Its history runs deep in the world of color, providing a backbone for certain hair dyes and acrylic-melamine resins, or becoming a key step along the road to making photographic developers. The groups placed side by side create a molecule that can be fine-tuned to react or bind with metals, something biology borrows on occasion for enzyme research and synthetic work.
That real-world use ties back to laboratory safety, too. The structure’s reactive sites call for caution — gloves, good ventilation, and a steady hand. Nitration, at that ortho position, gives fast access to many derivatives, an advantage for folks chasing a specific molecular property. Bringing these derivatives into commerce requires understanding where accidental oxidation might threaten safety, and how to control for purity. These aren’t textbook worries; they pop up every year in plants and small research labs alike, and controlling those reactions means fewer spills and dangerous surprises.
Anyone storing 2-aminophenol for a project needs to think about stability. Exposure to air can give rise to slow oxidation. As it degrades, color shifts appear, sometimes signaling bigger troubles inside a storage bin. Sealed bottles, stored cool and dry, tend to give the longest shelf life. Laboratories working with open vessels see color changes quicker. This isn’t just cosmetic; impurities creep in, reactions get unpredictable, and analysis results skew away from the truth. In pharmaceutical synthesis, this unpredictability can turn costly or dangerous, so handling and reporting must stay sharp.
Old chemistry lessons stressed rote memorization of formulas and names. In this case, though, learning why the -NH2 and -OH groups make a difference helps bridge the gap between theory and the real world. Chemists, plant operators, and environmental specialists use that structure to predict how 2-aminophenol behaves in soil, water, or the lungs of a person exposed during production. Insisting on safe storage, watching for byproducts, and designing newer, safer dyes or antioxidants often calls for revisiting the nuts and bolts of molecules like this.
2-Aminophenol belongs to the family of aromatic compounds and plays a big part in chemical manufacturing. It finds a place in making dyes, pharmaceuticals, and sometimes in photography as a developer. This chemical, with both an amine and a hydroxyl group, shows up often in labs and factories. That said, questions about its hazards and toxicity should never be swept aside just because it is useful.
Take it from anyone who’s worked with this chemical: its powder or crystals look harmless, but contact isn’t something folks should shrug off. Even at low doses, 2-Aminophenol can irritate the skin, and direct handling without gloves leads to redness and soreness pretty quickly. It’s not just skin—the eyes sting after a splash, and even vapor can bother upper airways. Inhaling dust produces coughing and discomfort, sometimes leading to headaches. It’s easy to avoid these troubles with proper personal protective equipment, but skipping safety gear has clear consequences.
Serious problems appear if 2-Aminophenol enters the body in large amounts or regularly over time. Studies in animal models document effects that shouldn't be brushed off—stomach pains, nausea, and even effects on the blood, such as methemoglobinemia (where oxygen flow in the body gets limited). A handful of occupational case reports connect extended, unprotected exposure to symptoms like dizziness, fatigue, and in rare cases, kidney or liver strain. Chronic exposure hasn’t shown clear links to human cancers, but no one working with this material skips basic safety for good reason.
A bottle of 2-Aminophenol on a bench isn’t a disaster waiting to happen—danger grows only if people ignore the basics. Fume hoods, gloves, and safety glasses block most routes of exposure. If dusts build up in the air, workers need ventilation, and storage should be dry and well-labeled. People who work with chemicals long-term can tell you that good habits matter more than luck. Ignoring headaches or sore throats, especially in settings with regular chemical handling, often leads to bigger problems later on.
Disposal and leaks often remain an afterthought, but not with this compound. Spills running into waterways spell trouble for aquatic life. Fish and small invertebrates absorb aromatic amines, disrupting growth and reproduction. Facilities with strong waste management standards keep 2-Aminophenol from making its way down drains. Responsible companies monitor effluent and use proper incineration, reducing risk to plants, animals, and surrounding communities.
Modern chemistry relies on both innovation and responsibility. A common lesson from occupational health is that a hazardous material loses most of its risk through attention and respect. 2-Aminophenol doesn’t rank among the most toxic industrial substances, but it still demands care. Training, labeling, good ventilation, and accessible safety data sheets all support a working culture where harm stays rare. The aim isn’t to spread fear, but to reinforce that caution in the lab makes sure everyone gets home in one piece.
Anyone who has worked in a lab knows the day only runs smoothly if everything is in its place and chemicals don’t spring surprises. 2-Aminophenol isn’t exactly a household name, but it plays a big role in developing dyes, pharmaceuticals, and some rubber chemicals. It looks like a white or reddish solid and brings with it a distinct, slightly unpleasant odor that lingers. It sounds harmless on paper, but anyone who has gotten even a whiff or a speck of dust in the eyes remembers the lesson well: this chemical packs a punch, and a little caution goes a long way.
Humidity and light both mess with 2-Aminophenol. Exposing it to air slowly changes its color, which signals that its structure might be shifting in ways that compromise experiments or products. Keeping it in a tightly sealed container—preferably one made of glass or high-quality plastic—prevents unwanted chemical changes and keeps your work reliable. Left on a bench or under poor lids, 2-Aminophenol draws in moisture and reacts faster with air. That means clumping, stickiness, or even hazardous byproducts, and nobody wants any of that.
Direct sunlight hits this compound hard, so a dark cabinet gives better results than a crowded shelf next to a window. That rule goes hand in hand with storing it away from heat sources. Think of the chemical shelf as an extension of your fridge for perishable food—keep it cool, dry, and away from things that react easily.
Leaky containers send odors through a room, and this is more than an annoyance. Some people develop headaches or breathing problems from repeated contact with these fumes. Skin exposure leads to irritation or rash, and eye contact stings more than most are ready for. In practice, using gloves and goggles matters more than people realize. I once witnessed a colleague rub his eyes after handling 2-Aminophenol— a rookie move that resulted in a trip to the wash station and a red-eyed morning. Respecting personal protective equipment (PPE) isn’t just box-ticking: it saves skin, sight, and hours lost on recoveries.
Spills happen no matter how tidy the workspace looks. Cleaning powders or granules with a dry cloth just moves the particles around or throws them in the air. Use a vacuum with a HEPA filter or sweep up carefully with damp materials, and always ventilate the area. These steps mean fewer headaches and safer shifts, especially for those who spend long hours in the lab.
Lab managers could support safer habits by posting clear instructions near storage areas. Routine checks a few times a year catch cracked lids or expired stock before anything turns ugly. Training newcomers isn’t about reciting hazard symbols; it’s walking them through safe habits and showing how easy it is to avoid accidents. Every extra minute spent sealing a cap or checking a label pays off tenfold in fewer emergencies.
The big principle here: chemicals belong in their proper place, with protective equipment always within arm’s reach. Anyone who cuts corners today may find themselves cleaning up a dangerous mess tomorrow. Dealing with 2-Aminophenol reminds you that old habits matter— and safety is always worth the effort.
Talk to anyone who’s worked in fabric dyeing or ink production and you’ll hear how much chemistry shapes the final product. For years, 2-Aminophenol has helped companies bring color to everything from textiles to receipts. It’s one of those compounds that turns a blank piece of fabric into a bold, lasting shirt or towel. You might not notice it at the laundromat, but behind that navy-blue work uniform lies a chemical story.
Look at thermal paper—like the receipts shoppers collect on a busy weekend. 2-Aminophenol helps shape the thermal coatings that make those letters appear at the register. It doesn’t stop at clothing and receipts; its reach in the color world runs deep, even in certain permanent markers and printer inks found at office desks.
Many everyday medicines rely on chemical building blocks that remain mostly hidden to patients. 2-Aminophenol gives pharmaceutical chemists a way to start assembling drugs for things like pain relief and infections. It can help build molecules similar to paracetamol—a painkiller found in millions of homes. Researchers value its reliability and versatility, which lets them pursue new compounds without major hurdles.
Pharmaceutical labs often need materials that don’t break down too soon or react in unpredictable ways. 2-Aminophenol’s structure makes it a solid starter for a family of medicines, including certain antipyretics and antimicrobials. Plenty of scientific articles stress how easily it helps form larger, more complex drug molecules.
Before everything turned digital, photographers trusted developers to turn exposed film into visible images. 2-Aminophenol showed up in some of those developer formulas. It’s the sort of role that, while rarely discussed outside specialized circles, mattered for generations of photographers. Even now, artists who still use film—myself included—find it in tools that keep traditional processes alive. It plays a part in the chemistry that brings black-and-white photos out of the darkroom and onto the wall.
Beyond the color and drug industries, 2-Aminophenol shapes complex molecules used in specialty chemicals. Working in an industrial lab, I saw it contribute to additives for lubricants and corrosion inhibitors. Industrial engineers need materials that can handle pressure and temperature changes. The resilience of these finished products can trace back to the starting chemistry, and 2-Aminophenol supports the stability engineers count on, whether it’s in gearboxes or engines.
In electronics, some manufacturing teams use it to build chemical components for circuit boards. Its ability to integrate into more advanced molecular designs helps the industry make devices that last longer and perform better. The electronics sector, famous for small margins and strict safety checks, doesn’t gamble with quality. Reliable raw materials like 2-Aminophenol help maintain those standards.
Talking about chemicals always means thinking about safety. Exposure in factories requires real oversight. Workers must use gloves, masks, and good ventilation, because studies show unnecessary contact can cause irritation and health risks. On the environmental front, proper storage and waste controls go a long way. Clear training and updated protocols make a difference—something I’ve pushed for after seeing the risks in action.
People do best with transparent labeling and routine monitoring. As technology advances, new alternatives could cut down on environmental impact. For now, awareness and responsibility stand tall as the most useful tools in staying safe and preserving our communities.
| Names | |
| Preferred IUPAC name | 2-Aminophenol |
| Other names |
2-Hydroxyaniline o-Aminophenol Orthaminophenol |
| Pronunciation | /tuː əˌmiːnəʊˈfiːnɒl/ |
| Identifiers | |
| CAS Number | 95-55-6 |
| 3D model (JSmol) | `/usr/share/jmol-2.5.6/JmolData/2AP/2-aminophenol.xyz` |
| Beilstein Reference | 1209229 |
| ChEBI | CHEBI:17703 |
| ChEMBL | CHEMBL1407 |
| ChemSpider | 579 |
| DrugBank | DB03851 |
| ECHA InfoCard | 100.032.404 |
| EC Number | 205-481-9 |
| Gmelin Reference | Gmelin107052 |
| KEGG | C01426 |
| MeSH | D011611 |
| PubChem CID | 5801 |
| RTECS number | BP8925000 |
| UNII | 83HN0GTJ6D |
| UN number | UN2512 |
| Properties | |
| Chemical formula | C6H7NO |
| Molar mass | 109.13 g/mol |
| Appearance | White to faintly pink crystalline solid |
| Odor | ammonia-like |
| Density | 1.293 g/cm3 |
| Solubility in water | Moderately soluble |
| log P | 0.62 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 9.76 |
| Basicity (pKb) | 9.74 |
| Magnetic susceptibility (χ) | -0.6×10^-6 cm^3/mol |
| Refractive index (nD) | 1.682 |
| Viscosity | 2.04 mPa·s (25°C) |
| Dipole moment | 1.310 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 95.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −40.9 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2341 kJ/mol |
| Pharmacology | |
| ATC code | D05BB03 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause allergic skin reaction, harmful if inhaled. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313, P362+P364 |
| Flash point | 102 °C |
| Autoignition temperature | 130°C |
| Explosive limits | Explosive limits: 2.8–17% |
| Lethal dose or concentration | LD50 oral rat 375 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 670 mg/kg |
| NIOSH | BZ8425000 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 200-500 mg/L |
| Related compounds | |
| Related compounds |
2-Nitrophenol 4-Aminophenol Aniline Catechol |