My time studying organic chemistry always led me back to industrial roots. In the late 1800s, chemists noticed that crude products from coal tar held several methylphenols. Among them, 2,6-xylenol stood out for its strong phenolic smell and its double methyl groups nestled on the benzene ring. Research teams in the 1920s started isolating and purifying this compound, moving from simple distillation methods to more refined approaches like fractional crystallization and solvent extraction. As the chemical industry expanded after the Second World War, demand for synthetic intermediates grew. By the 1950s, 2,6-xylenol left the status of an academic curiosity behind, finding uses in new polymers and resins. Today, its applications stem from innovative needs, yet historical development never strayed far from its coal tar beginnings.
2,6-Xylenol, which many call 2,6-dimethylphenol, serves as a versatile phenolic compound. In personal experience, it plays a major part when producers want high-performance polymers. Its strong, slightly medicinal odor acts as a sensory warning of its reactive nature. Many manufacturers rely on 2,6-xylenol as a starting block for polyphenylene oxide (PPO) resins, thanks to its methyl layout. Each year, thousands of tons move through chemical plants in Asia and North America, proving how it has become a commodity as much as a specialty chemical.
With a melting point close to 110°C and boiling above 200°C, 2,6-xylenol resists easy vaporization or breakdown. In the solid state, white flaky crystals are common, and they sometimes pick up a yellow tinge after exposure to air. This compound dissolves well in alcohols and ethers, less so in water, due to its two methyl groups reducing hydrogen bonding. From my lab days, I remember its tendency to oxidize at room temperature, leading to color changes if left uncapped for weeks. Chemically, the methyl groups block some electrophilic attacks at the 2 and 6 positions. That selectivity helps chemists channel transformations toward the 4 position, giving flexibility in downstream syntheses.
Industrial supply houses expect 2,6-xylenol to meet high purity standards, usually above 99.5%. Impurities like cresols or other xylenols can hinder production of high-quality polymers. Drums and containers usually hold a standardized label: chemical name, purity percent, hazard pictograms for flammability and toxicity, CAS number 576-26-1, batch number, and QR code for digital tracking. Such details may seem dry, but safety teams rely on precise labeling to avoid mishaps, given the compound’s toxic and caustic nature.
Most production today follows a methylation route, starting from phenol and methylating agents such as methanol, with catalysts like aluminum oxide or zeolites to guide selectivity. In one of my previous projects, the key challenge came down to adjusting catalyst beds for optimal temperature and pressure. This kept yields high while reducing undesirable byproducts. Some smaller manufacturers still draw from historical routes—tracking back to coal tar distillation and high-resolution separation—but synthetic routes from basic aromatics have dominated since the late 1970s. Waste management, especially proper venting of volatile organic compounds and treatment of methanol-rich effluent, often decides the environmental footprint of a production plant.
Reactivity of 2,6-xylenol invites opportunities for chemical modification. Laboratory teams frequently run oxidative coupling, producing polyphenylene oxide (PPO)—a sturdy thermoplastic. Halogenation targets the remaining ring positions, often producing intermediates for herbicides or flame retardants. Methyl groups shield parts of the aromatic core, granting synthetic chemists fine control for multi-step sequences. From personal trials, I found that alkylation and acylation occurred most cleanly in polar aprotic solvents, as water suppressed reactivity. Modern green chemistry pushes for catalysts that operate under milder conditions and with less environmental burden, a trend that’s only gaining strength year by year.
2,6-Xylenol sometimes shows up as 2,6-dimethylphenol or even DMP among engineers. Other naming conventions include adding “phenol” before “dimethyl” to avoid confusion with xylene derivatives. Packaging for polymers might call it a PPO precursor. Distributors also trade under trade names tied to purity or region (for example, XyloPure 99). In procurement work, attention shifts to the CAS number 576-26-1 and global inventory IDs rather than just brand names, to guarantee consistent supply for large batch runs.
Anyone working with 2,6-xylenol deals with a chemical that irritates skin, eyes, and respiratory tract. Toxic vapors escape readily, so proper ventilation makes a difference between a safe and hazardous workspace. I once observed a near-miss where gloves failed inspection, allowing skin contact and subsequent burns. Current operational norms require full PPE: gloves, goggles, face masks, flame-retardant lab coats. OSHA and EU REACH regulations establish exposure limits and emergency handling protocols, blending decades of workplace experience into clear rules. Emergency showers, eyewash stations, and chemical spill kits remain non-negotiable features in every facility storing this compound.
Polyphenylene oxide (PPO) resins absorb a lion’s share of global demand, especially for circuit boards, electrical insulators, and automotive plastics. High-temperature stability and low moisture absorption give these polymers a performance edge. Producers of pesticides, antioxidants, and UV absorbers use 2,6-xylenol as a base material. Over the years, medical and pharmaceutical research explored its antimicrobial potential, though toxicity concerns restrict use in consumer-facing health products. Its specialty derivatives serve as flame retardant intermediates, each modification tweaking solubility or reactivity according to a niche request from electronics or coatings sectors.
Ongoing R&D looks for greener production methods and new catalysts to cut both cost and environmental impact. Academic labs test bio-based routes, aiming to replace petroleum feedstocks. Success remains partial—yield, selectivity, and purity gaps persist between bio-routes and tried-and-true synthetic processes. Industry often funds joint research into high-efficiency reactors and inline real-time monitoring, trying to pinpoint impurities before they gum up large-scale reactions. Intellectual property battles sometimes erupt over the tiniest tweaks to process or application. My conversations with process engineers suggest that scaling up remains a sticking point, particularly as demand for higher-grade PPO grows in data-center and renewable energy infrastructure.
Toxicologists scrutinize 2,6-xylenol due to its acute toxicity on skin and through inhalation. Animal studies show rapid absorption and metabolic breakdown in the liver, with nephrotoxic and hepatotoxic effects at high doses. Chronic exposure links to nervous system symptoms. From a regulatory perspective, agencies assign tight permissible exposure limits and call for biomonitoring in industrial plants. My own read of the literature highlights gaps in understanding long-term effects from environmental exposure—especially waterborne discharge near chemical plants—making further toxicological assessment a clear priority.
Consumption of 2,6-xylenol tracks the growth of high-performance plastics. As the world leans harder into electric vehicles and smart infrastructure, polymer manufacturers forecast steady demand. Transition toward cleaner production and tighter emission controls will guide next-generation plant design. I see potential in closed-loop recycling, where used PPO resins revert to monomers through chemical depolymerization, feeding right back into the supply chain. Innovations in biocatalysts and continuous-flow systems push boundaries, aiming for sustainability and efficiency. Environmental and workplace safety concerns will persist as watchdogs raise the bar, but ongoing research—and daily vigilance in the lab—drive the field toward safer and smarter breakthroughs.
2,6-Xylenol, known to some folks as 2,6-dimethylphenol, pops up in more places than you might expect. Most people never hear the name, yet it plays a role behind the scenes in everyday products and industrial materials. A colorless solid at room temperature, it blends strong chemical traits with flexibility, making it vital for certain manufacturing processes.
Experience in manufacturing shows that 2,6-Xylenol stakes its claim most noticeably in the world of plastics. One crucial material, polyphenylene oxide resin (PPO), depends on it. This resin mixes into blends like Noryl, which you find in power tool housings, automobile parts, and electrical equipment. These plastics tolerate heat, stand up to impact, and don't easily get scratched, so gadgets stay working even when conditions aren't friendly.
It's one thing to talk about durability, but in reality, companies choose PPO resins because they keep shape and structure even near engines or in hot climates. Without a steady supply of 2,6-Xylenol, engineers would lose a key piece of the puzzle in designing safe electronic casings and car parts. For those working in electrical shops or assembly lines, 2,6-Xylenol's influence is visible every day—even if tucked away inside a product label.
Beyond plastics, 2,6-Xylenol finds its way into specialty antioxidant blends. Manufacturers create certain antioxidants using 2,6-Xylenol to protect rubber and plastics from breaking down when exposed to heat or light. Think about car tires. They get roasted in the summer sun and flexed around sharp turns, yet hold together for years. Rubber and plastics last longer with the help of chemicals like 2,6-Xylenol. Its presence keeps materials from turning brittle, which means less waste and fewer replacements.
This field isn’t about convenience. It’s about making sure products survive real life. Everyone’s seen garden hoses that dry up and fall apart by the second summer. Imagine if all rubber cracked as fast. Choosing right additives keeps those items useful longer, which helps both customers and companies spend less money over time.
Some pharmaceutical products also draw on 2,6-Xylenol. It acts as a starting material for synthesizing medicines and chemical intermediates. For instance, a few antiseptics for skin rely on derivatives made from 2,6-Xylenol. Factories use it not because of any magic, but due to how it reacts and the options it opens up for modifying molecules. As new treatments come along and impurities demand stricter controls, chemists lean on trusted chemicals like this to build compounds step by step.
Working with 2,6-Xylenol reminds me that chemistry, at its core, balances innovation against caution. It lets us push boundaries in making safer cars, sturdier power tools, and longer-lasting goods. Its uses bring better performance, but also shine a light on the need for careful handling during production and disposal. Breathing in lots of dust or fumes can cause headaches and irritation. Protecting workers and the environment takes steady focus—proper ventilation, sealed systems, and routine checks all matter.
Finding safe substitutes or recycling old polymers stands as a useful challenge for the chemical industry. For now, 2,6-Xylenol serves as a quiet workhorse. Choosing responsible practices today shapes how manufacturers, workers, and communities share in the benefits tomorrow.
Everyday chemistry often hides its most useful players in plain sight. 2,6-Xylenol holds a spot among them. Also known as 2,6-dimethylphenol, this compound features a benzene ring, which forms the backbone for a huge number of chemicals in both industry and research. Its chemical formula, C8H10O, speaks to its simplicity and versatility. The magic sits in its configuration: two methyl groups set up shop on the second and sixth carbons of the ring, with a hydroxyl group (–OH) at the first carbon spot.
Looking at a structural formula helps: Picture a hexagonal benzene ring. One corner has an –OH group, skipping a spot on either side before a methyl group (–CH3) pops up at both positions 2 and 6. The rest of the ring carries plain old hydrogen atoms. That kind of precise placement means this isn’t just any phenol. The presence of those methyl groups has a real impact on how this molecule behaves in chemical reactions, changing both its physical properties and its relationships with other chemicals.
Chemically speaking, grouping methyl and hydroxyl units on a phenol ring tweaks things like solubility and reactivity. This arrangement makes 2,6-xylenol more soluble in nonpolar solvents and more resistant to certain oxidative reactions than phenol itself. Factories rely on those exact traits. I’ve seen it used as a key intermediate in the production of polyphenylene oxide (PPO) resins – strong plastics that show up in parts for electronics and automobiles. Engineers prize these plastics for their stability when faced with heat or electric currents. They turn to 2,6-xylenol, because its structure gives PPO a backbone that stands up to wear and tear.
I once shadowed a team using 2,6-xylenol to create specialty resins for electronics. Getting the right mix called for more than just knowing the formula – understanding the precise connections between atoms let the chemists work out how much heat and pressure the final product could take. That hands-on knowhow is shaped by a deep grasp of structure, not just by looking at a list of ingredients.
Handling 2,6-xylenol safely carries weight, both for chemists and for larger communities. This chemical can be toxic to aquatic life, and its vapors may cause respiratory irritation. Regulators like the EPA encourage proper containment, and it makes a lot of sense for companies to keep emissions as low as possible—nobody wants pollution in the water table, and everyone deserves clean air. Modern plant systems now use scrubbers and recycling loops to capture or reuse vapors, slashing waste and risk side by side.
Looking forward, research circles also focus on greener synthesis routes. Catalysts have received plenty of attention. Some groups explore the use of enzymes or more selective metal catalysts that operate under lower temperatures and pressures, reducing the carbon footprint. Cleaner and safer manufacturing steps aren’t just about regulatory compliance; they're about future-proofing a material that many industries lean on.
2,6-Xylenol’s value lies right in its arrangement of atoms. Its formula stays short, but its influence stretches far, tied to industries and innovation. Recognizing those links opens the door for more responsible, resourceful chemistry in production and stewardship.
A lot of everyday people haven’t heard of 2,6-xylenol, though it lurks behind plenty of industrial uses—think plastics, resins, possibly even some disinfectants. It shows up in places you'd never expect, like the coatings on your electronics or in adhesives holding household things together. But this chemical doesn’t just work quietly in the background. Scientists and workers share stories about the risks it brings, especially when folks handle it with their bare hands or breathe in its fumes day in and day out.
You can’t ignore the effect that 2,6-xylenol has on workers. Plenty of lab reports point out skin and eye irritation, breathing issues, and possible long-term impacts on the liver and kidneys. Years ago, I worked near a plant that used phenolic chemicals, and nobody there took mask-wearing lightly. Their union had fought long and hard for fresh air and gloves, telling anyone who’d listen about dizziness or sore throats picking up after a shift. Peer-reviewed studies echo their concerns: short-term exposure burns or blisters the skin, and nearby air can see big spikes in volatile organic compounds (VOCs). In larger doses, animal studies hint at trouble for the central nervous system, suggesting that heavy, repeated contact brings on more lasting health hazards.
Dumping 2,6-xylenol can poison local streams or groundwater. This stuff doesn’t just disappear when washed down the drain; it lingers, tough to break down, and builds up. If it leaks from storage tanks or runaway manufacturing, fish and frogs pay the immediate price. I’ve read field reports showing soil contamination in areas surrounding chemical plants, upending farming efforts and scaring off families who depend on their land. In aquatic settings, it can take weeks or months before sunlight and bacteria break it down, giving time for wildlife to suffer or die off.
OSHA and the EPA don't take 2,6-xylenol lightly. Both agencies set strict limits, keeping workplace vapor levels low and demanding cleanup for spills. Still, slip-ups and shortcuts happen. In 2019, a spill in the Midwest put the spotlight back on the chemical, forcing local officials to shut down a stretch of river and distribute bottled water for days. Looking deeper, the Agency for Toxic Substances and Disease Registry links similar phenolic compounds to waste site problems, with long-lasting effects on everything from plant roots to human chromosomes.
Better air monitors, smarter chemical storage, and clear emergency drills help make a difference. Tools like closed-system handling and full-face respirators aren’t just wish list items—they’re shields for the workers and neighbors who pay the price otherwise. Training also makes a dent. My cousin, who runs safety workshops for chemical transporters, says honest, straight talk about real accidents grabs attention far better than dull rulebooks. Every company dealing with 2,6-xylenol ought to invest in routine health checks for its staff, giving folks early warning about exposure before symptoms show.
Switching to friendlier compounds in manufacturing can lighten the load this chemical puts on the environment. Researchers have flagged alternatives with lower toxicity, and pushing for these options offers manufacturers the chance to protect both people and profit. Rules matter, but culture and leadership at every plant matter even more.
Ignoring 2,6-xylenol’s risks won’t make them disappear. We see proof in headlines and health data. Companies, regulators, and community groups all have skin in the game. It takes commitment, information, and better safeguards to break the cycle of risk. With genuine action, we can keep both people and local habitats healthier and safer in the chemical age.
2,6-Xylenol shows up across a bunch of industries—resins, antiseptics, dyes, and everyday adhesives. At first glance, this white, crystalline solid doesn’t look much different from ordinary chemicals cluttering up the back of a lab shelf. A mistake like that can bring on a world of trouble. A brief whiff can irritate lungs, skin contact starts burning, and spills sneak up on clothes and shoes.
In chemical labs, even seasoned technicians treat 2,6-Xylenol with respect. Once, during an internship, I watched a careless glove change lead to a mild chemical burn. The lesson stuck: gloves aren’t just for show. Nitrile or neoprene offers decent protection. Cotton and latex won’t cut it. Double-gloving isn’t overkill. Once those gloves come off, washing hands remains a non-negotiable habit. Safety goggles protect against stray dust, which pops up when opening up drums or scooping the powder. A splash in the eye puts vision at risk, so face shields join the lineup during bigger pours.
Nobody likes the sting of inhaling sharp fumes. 2,6-Xylenol easily produces vapors above room temperature, so working in a well-ventilated fume hood saves headaches—literally. Central ventilation keeps trouble out of breathing space. Any spills need sealing off right away, not just mopping up with a rag and hoping for the best. Scooping up solids, then dousing remains with sand or a spill kit, avoids fire hazards.
This compound sets off easily. Tossing used rags near heat is asking for a fire. Closed, marked containers live in cool, dry rooms, far away from flames or oxidizers. People sometimes get lazy, stacking drums too close or forgetting secure lids. Those shortcuts raise risks fast.
Direct contact—skin, eyes, or breathing in dust—feels rough and can land someone in hospital. Symptoms look like burning skin, eye swelling, or harsh coughing. Flushing with water helps a lot, but contaminated clothes need to come off quickly. Calling medical services shouldn’t wait if someone’s breathing feels weird or if eyes get hit. Time matters with chemical injuries—putting off treatment means more trouble.
Too often, unfamiliarity leads to mistakes. Proper training for everyone around this chemical builds safe habits. Reading safety data sheets before entering the workspace sets clear expectations. Not everyone knows that dry towels ignite easier than damp ones, or that vapor can linger in small rooms for hours. Small workshops need written plans, just like big factories.
Studies from the National Institute for Occupational Safety and Health have mentioned 2,6-Xylenol’s ability to irritate the respiratory tract even at low levels. Workers reporting dizziness or nausea provide a red flag for ventilation issues. The American Conference of Governmental Industrial Hygienists lists a threshold of just 2 ppm as a time-weighted exposure limit.
Sensible rules—fresh gloves, working in well-aired spaces, and daily checks on ventilation—carry the workforce further than any warning sign on a cabinet. Clear emergency instructions posted near storage areas take out panic from the equation during spills or exposure. Swapping everyday cleaning rags with proper spill materials and never working alone with 2,6-Xylenol round out the approach.
Chemicals with bite like 2,6-Xylenol serve a role, but safety grows out of honest habits and consistent training, not luck. Safe handling means watching each step, not skipping out on the basics. The extra time pays off by letting everyone head home in one piece.
2,6-Xylenol forms part of many industrial products. I remember seeing it listed in adhesives and even in resins. This chemical acts as a strong irritant and can harm the body if inhaled or if it gets on your skin. I follow chemical safety news, and accidents often happen because someone cut corners or failed to plan for emergencies. Keeping 2,6-Xylenol secure and disposing of it properly means protecting not only your health but also the water you drink and the land under your feet.
Direct sunlight, heat, and moisture turn this chemical into a bigger hazard. I store anything like this in a dry, shady spot, away from heat sources and sparks. Flammable vapors build quickly. Use containers made of glass or specific plastics that resist corrosion, not just any old bottle found in the lab. Labels help—every bottle in my workshop carries a date and a clear name.
You keep spills from spreading by sealing the containers tight after each use, and never mixing leftovers. Stacking them next to acids or oxidizers only increases the risk of a reaction. A friend once shared a story about a nearly tragic mix-up in a crowded storeroom. A dedicated storage area with signs and a simple spill kit close by goes much further than a long safety manual sitting on a shelf.
Anyone who handles industrial chemicals wears gloves and goggles, plain and simple. Splash-proof lab coats and chemical-resistant gloves stop burns and rashes. I know one colleague who learned the hard way after a tiny drop landed on his skin; it only took a minute to blister. Breathing protection makes sense if the room doesn’t have air movement; these vapors can knock you out.
Work with this kind of product in a well-ventilated area or under a fume hood, not in the broom closet or beside the break room. A clear emergency plan should not be an afterthought—a phone list and an eyewash station save lives.
Many people think pouring small amounts down the drain causes no harm. Once, during a site visit, I saw a technician dump spent chemicals into the sink. The look on my supervisor’s face said it all. Water treatment plants can’t remove many hazardous compounds. Tiny amounts can poison fish and foul the water supply.
Contact a hazardous waste company for chemical pickup. These pros understand the regulations and have gear to deal with the risks. Before handing anything over, place containers into a secondary leak-proof bin for transport, and double-check the labels. Mixing different chemicals in the same waste stream will make disposal harder, not easier.
Each time a business or school stores and disposes of chemicals the right way, it prevents fines and health scares. It also sets a standard for everyone who works or studies there. Choosing safety keeps our air and drinking water cleaner. Teaching others about safe habits takes a little time, but the habits stick and spread across generations. It boils down to respect—for your own body and for your neighbors.
Storing and disposing of 2,6-Xylenol with attention and care means fewer emergencies, less harm, and a healthier community.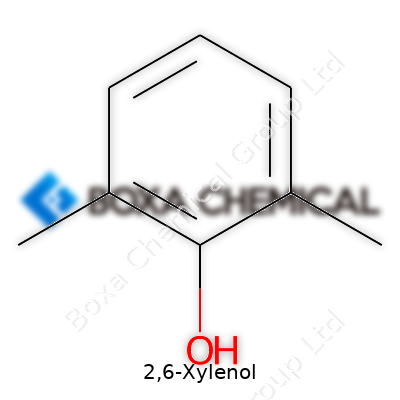
| Names | |
| Preferred IUPAC name | 2,6-dimethylphenol |
| Other names |
2,6-Dimethylphenol 2,6-DMP |
| Pronunciation | /tuː,sɪks-zaɪˈliːnɒl/ |
| Identifiers | |
| CAS Number | “576-26-1” |
| Beilstein Reference | 1209227 |
| ChEBI | CHEBI:13606 |
| ChEMBL | CHEMBL14170 |
| ChemSpider | 5537 |
| DrugBank | DB11396 |
| ECHA InfoCard | ECHA InfoCard: 100.003.250 |
| EC Number | 1.10.3.2 |
| Gmelin Reference | 120209 |
| KEGG | C01783 |
| MeSH | D015850 |
| PubChem CID | 13733 |
| RTECS number | ZE4500000 |
| UNII | YG6YF29NOF |
| UN number | UN2430 |
| CompTox Dashboard (EPA) | DTXSID7021269 |
| Properties | |
| Chemical formula | C8H10O |
| Molar mass | 122.17 g/mol |
| Appearance | White crystalline solid |
| Odor | Phenolic |
| Density | 1.03 g/cm3 |
| Solubility in water | 10.7 g/L (at 20 °C) |
| log P | 2.2 |
| Vapor pressure | 0.28 mmHg (25°C) |
| Acidity (pKa) | 10.6 |
| Basicity (pKb) | 9.91 |
| Magnetic susceptibility (χ) | -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.528 |
| Viscosity | 6.35 mPa·s (25 °C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 137.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −47.69 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3565.0 kJ/mol |
| Pharmacology | |
| ATC code | D02AE02 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 3*2*0* |
| Flash point | 129°C |
| Autoignition temperature | 530°C |
| Explosive limits | 1.1–1.1% |
| Lethal dose or concentration | LD50 oral rat 795 mg/kg |
| LD50 (median dose) | LD50 (median dose): 795 mg/kg (oral, rat) |
| NIOSH | ZE2450000 |
| PEL (Permissible) | 50 ppm (Ceiling) |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 50 ppm (NIOSH, 2024) |
| Related compounds | |
| Related compounds |
Phenol cresols 3,5-xylenol 2,4-xylenol |