Chemists in the late nineteenth century eyed aromatic quinones with curiosity, and 2,6-naphthoquinone got its place in research thanks to its clear structure and reactive oxygen atoms. Early researchers, aiming to unravel the mystery of oxidative dyes and pigments, quickly found naphthoquinones harder to handle than their benzoquinone cousins. Following the industrial revolution, chemical manufacturers needed robust molecules for colorants and intermediates. In the decades after, 2,6-naphthoquinone gained traction as scientists learned to fine-tune those double bonds and carbonyl groups, fitting them into dye manufacture, redox chemistry, and beyond. The journey of 2,6-naphthoquinone through labs and factories reflects people’s need to understand and harness chemical reactivity for goods that eventually shaped daily life, from colored textiles to the next generation of specialty chemicals.
2,6-Naphthoquinone, often recognized in production lines and research facilities, shows up as a strong yellow crystalline solid. This compound carries real value for those working on dyes, intermediates, batteries, or even exploring antioxidants in biological studies. Known by other names like 2,6-dioxonaphthalene or simply 2,6-NQ, it comes in standard packages from specialized chemical suppliers, with purity ranges high enough for both industrial and academic work. Manufacturers invest extra effort in quality control, since even slight impurities may alter downstream chemistry. Technicians and researchers have come to rely on its consistency for plenty of procedures, whether in organic synthesis or electroactive material development.
Sitting as a dense, yellow crystalline powder, 2,6-naphthoquinone holds a melting point around 271°C, which signals its stability under fairly demanding synthetic conditions. Its moderate solubility in polar organic solvents like dimethyl sulfoxide or acetone makes it flexible in most organic synthesis labs. In water, its solubility sharply drops — only trace amounts dissolve, which limits its use in purely aqueous applications. The compound showcases high oxidative potential, allowing for redox activity in many chemical transformations. Spectroscopic analysis reveals well-defined peaks for those carbonyl stretches, which lab analysts count on for quality assurance. The real-world meaning of these characteristics becomes clear in practical application, where operators handle robust processes and often push for efficient transformations and recoveries.
Regulatory and plant safety officers care deeply about product traceability and safety labeling for a chemical like 2,6-naphthoquinone. Standard packaging includes product name, chemical abstract number, lot number, net weight, date of manufacture, and any hazard classifications according to GHS standards. Detailed certificates of analysis travel with each batch, ensuring all users know the purity (usually exceeding 98%) and major impurity profiles. Storage advice appears directly on the labeling: keep containers tightly closed, away from direct sunlight and moisture, and handle in well-ventilated environments. For many users, this kind of straightforward transparency builds trust, lowers risk, and smooths regulatory inspections.
The most common method for producing 2,6-naphthoquinone relies on oxidation of 2,6-dimethylnaphthalene or 2,6-dihydroxynaphthalene using strong oxidants such as chromium trioxide, potassium permanganate, or other transition metal catalysts. Operational teams tend to prefer routes with lower environmental quotas, steering away from heavy metals when possible. Modern chemistry, aiming for green credentials, pivots to milder oxidants or newer electrochemical methods. Though these greener options still struggle to match classic yields, they point toward safer and more sustainable production. Lab-scale routes easily adapt to automated flow reactors, improving reproducibility and making scale-ups smoother.
2,6-Naphthoquinone acts as a mild oxidant and a springboard for a wide range of organic modifications. The reactive oxygen atoms draw in nucleophiles, making it possible to introduce substitutions directly on the aromatic ring system or to reduce the quinone into its hydroquinone form. Organic chemists often build off the quinone core to create more complex pharmaceuticals, dyes, and ligands. In redox processes, 2,6-naphthoquinone offers unique cycling between oxidized and reduced forms, relevant for battery and sensor development. The molecule’s symmetry and accessible reactivity set it apart: you can attach sulfonate groups, halogens, and even larger organic chains depending on the conditions, allowing tailored products for advanced applications.
In catalogs, 2,6-naphthoquinone may appear as 2,6-naphthalenedione, 2,6-dioxonaphthalene, or simply by the abbreviation 2,6-NQ. Some suppliers use trade designations, embedding the molecule in proprietary blends for specialty electronics or dye production. Researchers still gravitate toward its formal name to avoid confusion with other naphthoquinones, which can differ widely in both chemical reactivity and hazards. The ecosystem of synonyms reflects the blend of industrial jargon and rigorous academic naming practices — newcomers often spend time mapping these product IDs to the actual chemical structure at hand.
Strict handling measures surround 2,6-naphthoquinone due to both its oxidizing character and irritant properties. Plant operators require gloves, goggles, and lab coats, while dust control plays a big role in minimizing inhalation or contact. Emergency procedures and clear signage remain in place wherever the chemical comes into play. Material safety data sheets describe first aid and spill response in direct language: flush contact area with plenty of water, move affected personnel to fresh air, and secure proper ventilation. On a regulatory front, companies often integrate automated leak and dust detection, aiming to head off incidents before they escalate. Environmental health officers regularly monitor air and surface levels, since buildup can cause respiratory or skin irritation in exposed personnel. The drive toward safer synthetic methods — swapping out the harshest oxidizers or solvents — continues to improve workplace outcomes.
2,6-Naphthoquinone’s impact stretches across several major industries. Dyes and pigments manufacturers count on it as a backbone for organic colorants, drawn by those conjugated bonds and easy modifications. Energy storage experts explore its properties for next-generation batteries and redox flow cell applications, taking advantage of rapid and reversible redox transitions. In organic synthesis labs, the quinone shape acts as a scaffold for building heterocycles and pharmaceutical intermediates. Analytical chemists rely on the molecule in redox indicator systems, while its bioactivity has nudged pharmaceutical researchers to probe both antimicrobial and anticancer effects. Even so, usage patterns tend to track the push for new functionality — if electronic devices or sensors call for stable yet reactive organic material, companies start R&D programs anchored on naphthoquinone structures.
R&D teams spotlight 2,6-naphthoquinone as a springboard for developing advanced materials and small-molecule pharmaceuticals. The SMART approach—combine structure analysis, mechanistic understanding, application-based testing, rapid iteration, toxicity screening—anchors most efforts. Synthetic organic chemists expand the chemical landscape by fine-tuning substitutions around the quinone ring, all aiming to enhance selectivity for targeted reactivity or biological response. Interdisciplinary collaborations bring together synthetic chemistry with material science, letting developers push into molecular electronics, electrochemical devices, or even photoactive films. Published patents and scientific literature show plenty of momentum; dozens of new derivatives and applications emerge every year, many aiming to reduce environmental footprint or boost energy density in technical fields.
Toxicological profiles for 2,6-naphthoquinone take top billing for researchers and regulators. Animal studies show the compound acts as a moderate irritant to eyes and skin, with potential for respiratory effects on prolonged or repeated exposure. Data on chronic toxicity or carcinogenicity remain less complete compared to some other naphthoquinones, though precaution rules most facilities. Biodegradation studies trace the breakdown products in soil and aquatic systems, monitoring risks of environmental accumulation. Toxicologists keep pace with new research by recommending workplace exposure limits—often informed by both acute and chronic animal studies. Companies support safe use by emphasizing engineering controls, personal protection, and thorough training. A recurring lesson from safety research: consistent and open communication across teams lowers risks for both users and the environment.
Looking toward the future, many voices see 2,6-naphthoquinone stepping into bigger roles in green chemistry and sustainable technology. The molecule’s redox flexibility attracts battery developers, especially as the world pivots from fossil fuels to renewable energy and grid storage solutions. Advances in synthetic pathways may further lower the environmental impact, helping to meet stricter global regulations. Research groups keep eyeing new medical and agricultural uses, counting on the broad biological activity of quinone scaffolds. As project teams dig deeper into structure–activity relationships, even more finely tuned derivatives may emerge for specialty coatings, high-performance dyes, and next-generation pharmaceuticals. A practical lesson from all this: molecules with a proven industrial track record often offer up new surprises as technology marches forward, connecting time-tested chemistry to fresh innovation.
2,6-Naphthoquinone doesn’t make it into everyday conversation, but the work it does spreads wide. In chemistry labs, you'd see folks reaching for this solid yellow compound, relying on it to help spin up other molecules. Organic synthesis doesn't move forward without key pieces that let reactions unfold. 2,6-Naphthoquinone sits in that toolbox. Whenever I needed to make a certain ring system or alter the oxidation state of a compound during a project, the quinone structure stepped up.
Drug discovery teams keep exploring molecules that can stop illness in its tracks. Some core chemical structures crop up time and again in that hunt, and the naphthoquinone family carries real weight. Synthetic chemists looking to make anti-tumor or anti-infective compounds often look at 2,6-Naphthoquinone. Its ability to take or donate electrons means it can help tweak molecules for testing against cancer, bacteria, and even parasites like malaria. Studies published in journals like the “European Journal of Medicinal Chemistry” show how naphthoquinone derivatives create a backbone for these kinds of drugs.
Colors in textiles, plastics, and inks rarely come from nature anymore. 2,6-Naphthoquinone steps into the dye works, helping give molecules deep, stable colors. The double-bonded oxygen atoms in the quinone ring mean you get big shifts in color when conditions change. Factories rely on these chemical properties to make reds, yellows, and even blues that don’t wash out fast, and I’ve seen the difference myself in fabric swatches treated for resistance to weather and sun.
Laboratories use 2,6-Naphthoquinone as a chemical probe. It reacts with certain biological molecules, including amino acids, and produces a color change. Chemists harness that simple trick to flag the presence of different substances in a mixture. Being able to test for small amounts of vitamin K, for example, gets easier with such a tool. Speed and accuracy go up when you can spot colors instead of relying only on fancy instruments.
Every chemical that works well in industry and research can leave a footprint. Quinones can harm aquatic life, and their breakdown products aren’t always harmless. In my time working with industrial chemists, I saw the shift toward better waste handling growing stronger each year. Rules get tougher, and so does public pressure. Making sure factories stick to safe disposal methods helps keep rivers and soil free from build-up of these compounds. Fact-checking disposal regulations and investing in cleaner synthesis routes make a difference. Green chemistry calls for alternatives that give similar results without risking wildlife or water quality.
Training lab staff to respect 2,6-Naphthoquinone’s risks, wearing gloves, and keeping ventilation strong stops most direct health threats. Chemical companies invest in equipment that traps or destroys harmful vapors. Chemists push for reactions that avoid extra solvents or produce less waste, exploring routes that use enzymes rather than harsh reactants. As someone who’s handled quinones, knowing where they’ll end up has to be a priority before using them at scale. Funding for eco-friendly research and keeping industry honest with inspections both move the needle on safety and sustainability.
From cancer research to colorful shirts, 2,6-Naphthoquinone plays a role larger than its name might suggest. As science advances, chemists will keep searching for better ways to produce what we need—without loading the environment with new problems. Balancing progress with care always pays off, especially with chemicals as useful and potent as this one.
2,6-Naphthoquinone is an organic compound that makes its way into laboratories, chemical stocks, and some specialty manufacturing. A lot of people working around chemicals hear about quinones and think of risk, and for good reason—many of them can attack living tissue, whether by inhalation, swallowing, or skin contact. For anyone who spends time in a lab or around chemical warehouses, understanding the risk tied to a specific compound is more than academic—it’s about safety and health.
Pale yellow and crystalline, 2,6-naphthoquinone can seem innocuous. Experience shows looks can be deceiving. This chemical irritates the eyes, skin, and lungs. Even brief contact can trigger a burning sensation or lead to redness and swelling. Inhalation brings the risk of coughing, sore throat, or even more severe respiratory issues if exposure continues or dust levels increase. If someone ingests even a small amount, nausea, stomach pain, or vomiting can follow.
Data from the U.S. National Library of Medicine’s resources, like PubChem, point toward more serious effects with increased or repeated exposure. Studies in laboratory animals hint at the potential for organ damage, especially after long-term contact. Quinones, including 2,6-naphthoquinone, tend to generate reactive oxygen species. Over time, that oxidative stress can damage cells, especially in the liver or kidneys.
The danger from 2,6-naphthoquinone doesn’t end with direct human contact. Improper disposal matters. Pouring leftover solutions down the drain or tossing waste in regular trash threatens waterways and soil. Even low levels in runoff can impact aquatic life, posing risks to fish and plants based on the compound’s reactivity and persistence. In the long run, those changes ripple out, affecting agriculture, drinking water, and natural habitats.
In workplaces using this chemical—research labs or chemical manufacturing—hazards grow without tight controls. A single cracked container or spill may release dust or fumes, with workers bearing the brunt. Most safety officers rely on fume hoods, gloves, goggles, and lab coats, but no gear completely replaces prudent handling and discipline. In my time working in shared research facilities, I’ve seen the difference made by simple things: clear labeling, updated Safety Data Sheets on every bench, and regular, not occasional, staff training.
Strict labeling and chemical inventory audits go a long way. Always use the right personal protective equipment, and never underestimate how much even a quick splash or whiff can matter. Immediate clean-up for spills, proper waste containers, and avoiding mouth or eyes during handling are basic steps—but easy to skip if rushed or distracted. No shipment or synthesis deadline ever justifies cutting corners, especially with compounds proven to irritate or damage.
Beyond individual action, industry needs tighter oversight and better reporting whenever these compounds slip into waste streams or get substituted for less reactive chemicals without proper review. Regulations from the Occupational Safety and Health Administration (OSHA) and similar bodies exist, but enforcement can’t lag behind new science. If new research finds that 2,6-naphthoquinone carries chronic risks, those standards must adapt quickly, not just patch up past cases.
New research may reveal even more about the effects of quinones like 2,6-naphthoquinone in coming years. Personal vigilance, good workplace culture, and open conversation between researchers, safety officers, and the public help keep accidents rare and health preserved. Understanding hazard isn’t alarmism—it’s responsibility. Fact-based decisions, grounded in everyday practice, keep both people and the environment safer over the long haul.
Chemicals often bring up thoughts of complex lab setups and endless safety checks. 2,6-Naphthoquinone shows up in dyes, certain organic syntheses, and a few niche applications. Like a lot of lab chemicals, this compound calls for a little respect and a practical storage setup. Letting it sit out on a bench in an open bottle or in a sunny window won’t fly.
Storing 2,6-Naphthoquinone poorly brings a couple of headaches. Heat and direct sunlight make this yellow solid break down or change color, sometimes leading to weird by-products you don’t want in your next experiment. Humidity creeps in and clumps up powders, or, worse, helps them react with the air. If things go off, you waste material and money, not to mention risk safety in your lab.
Experience in university and industrial labs both taught me the value of a steady room temperature. 2,6-Naphthoquinone handles best at about 20-25°C. Some scientists stick it in a fridge, around 4°C, to slow down any slow-forming impurities, but extreme cold can sometimes draw moisture inside if you pull it out too often. A dedicated chemical storage cupboard away from heat sources strikes the best balance.
A sealed, tight cap on the original bottle makes a real difference. I can recall how a colleague’s open jar turned gritty after just a week in a humid storeroom. Silica gel packs inside the container, or simply storing the bottle in a desiccator, keep the air dry and prevent clumping or unwanted reactions. Keeping the powder off lab benches covered in spills goes without saying. Water and 2,6-Naphthoquinone don’t mix.
Sunlight or even strong overhead lights can slowly reduce the quality of 2,6-Naphthoquinone. Years ago, an old storeroom taught me this lesson. A sunlit shelf turned the top layer brown on a whole batch, making it useless for precision work. Amber glass bottles cut down UV exposure, but a dark cabinet brings even better results.
No matter how routine things get, sloppy labeling leads to trouble. Each container should show the name, date it arrived, and who opened it. I’ve seen sharp students pull the wrong naphthoquinone from a shelf, and the resulting mixup meant repeating an entire synthesis. Separate storage from incompatible chemicals—acids, bases, and especially reducing agents. Simple signs and divided shelves work better than fancy systems that get ignored.
Spills happen, even if nobody wants to admit it. A well-marked spill kit nearby, gloves, and goggles are key in every lab I’ve visited with a good safety reputation. The goal is quick cleanup and no panic. Aerosols and powders call for a gentle hand and damp towels, not frantic sweeping that stirs up dust.
Getting storage right isn’t just about ticking off a list for an audit; it’s about keeping research on track and people safe. Core principles—cool, dry, and dark—stand out. Clear labels, mindful separation, and thoughtful procedures round out a solid approach for anyone handling 2,6-Naphthoquinone regularly.
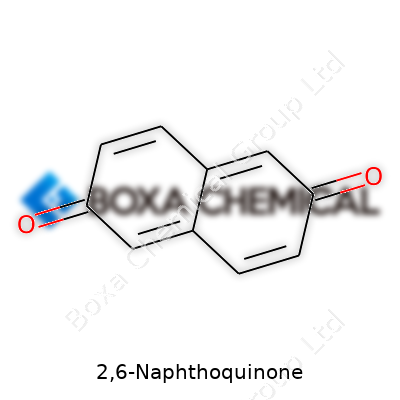
If you ever took apart the chemistry behind dyes, medicines, or certain industrial processes, you’ve probably seen names like 2,6-Naphthoquinone come up. The molecular formula for this compound is C10H6O2. This crisp, simple formula holds a lot of meaning. Let's dig into why that matters and where this knowledge can actually make a difference.
Chemical formulas aren’t just lines and numbers on paper. They shape how we build new compounds and understand reactions at a fundamental level. Back in the day, trying to synthesize even a tiny molecule without the correct formula meant working in the dark. I remember in my undergrad lab, mixing up molecules by formula alone got us in trouble – a single hydrogen off and the substance didn't even smell the same.
In the case of 2,6-Naphthoquinone, the exact sequence and type of atoms — ten carbons, six hydrogens, two oxygens — affects not just the color or structure, but every property from how it dissolves to how it reacts. Miss that oxygen, or shift a carbon, and you end up with a completely different compound that can't step into the shoes of the original. Chemistry in the real world is just unforgiving.
This specific molecule shows up in various industrial and research settings. It's used in the lab for organic syntheses and sometimes as a dye precursor. Studies have also looked at its biological activity — certain quinones, including close relatives, have demonstrated antibacterial or antifungal properties. Knowing the formula C10H6O2 lets researchers hunt for these effects efficiently, skipping wild-goose chases caused by guessing at structures.
Many chemists rely on accuracy. In the pharmaceutical field, molecular formulas help with regulatory filings and batch production. These days, regulatory agencies crack down hard if the paperwork doesn’t match up exactly with what’s in the flask. Getting the basic data right protects companies from compliance woes and consumers from unexpected risks.
For students, memorizing formulas means less time wasted in classes and more time actually understanding reactions and mechanisms. I found that setting aside extra minutes to draw the formulas by hand made other parts of organic chemistry click much faster. Teachers can help by linking molecules like 2,6-Naphthoquinone to real-world products—this grounds the abstract details in everyday use.
On the industrial side, digital catalogues and open-access chemical databases lower the barrier to getting reliable molecular data. This shift has been huge for labs that need to cross-reference or substitute compounds on the fly. By making sure everyone works with correct, clear formulas, miscommunication and costly mistakes get sidelined. Companies can focus less on basic checking, more on innovation.
Truth is, fundamental details like the molecular formula C10H6O2 unlock much broader possibilities in science, manufacturing, and education. Accuracy here isn’t just about paperwork; it keeps research moving and keeps the products we all use safe and trustworthy.
2,6-Naphthoquinone isn’t something people casually ask about at the grocery store. This chemical holds a place in industry, research, and sometimes art. It usually shows up in labs working on organic dyes, or in groups studying antioxidant activities in plants. Universities teach students about its roles in organic synthesis, though outside chemistry circles the name hardly comes up. At home, most folks never see it. This stuff is not a household cleaner or a go-to for hobbyists fiddling in the garage.
From a safety point of view, anything with “quinone” in its name deserves respect. Breathing dust or getting it on your skin can irritate more than just a science teacher’s patience. Labs handling it take health seriously; those rules matter for buyers too.
For those who need 2,6-Naphthoquinone, big chemical suppliers like Sigma-Aldrich, Fisher Scientific, or TCI America stand out. These companies vet their buyers. They need a business or school address, plus a reason for the purchase. Students can sometimes use their university accounts, as long as they have a green light from safety officers or professors. Businesses usually run requests through a purchasing department, coordinating paperwork with safety data sheets (SDS) included.
Most of the specialized chemical shops online don’t deal in single grams. Quantities and prices suit labs, not folks curious to try small-scale experiments. Any genuine vendor posts certificates of analysis. If a site looks too loose, skips paperwork, or avoids sharing SDS, trust drops fast. Sketchy sources risk more than expired stock; they can threaten safety or even violate laws.
In some cases, academic research teams team up and order in batches to save on cost and meet minimum orders. Working with your school or workplace brings better oversight too—no surprises with storage or waste disposal popping up later.
Handling specialty chemicals means dealing with rules. Laws in the US, UK, and across Europe steer who can buy and move quinones. Some countries keep an eye on certain chemicals, tracing shipments in and out of borders. Failing to comply carries real risks.
I once watched a small startup get stuck waiting weeks for a chemical, just because a key step in import documentation had slipped through. Nothing ruins a project deadline more than red tape. The lesson: always double-check licensing and customs, even for something a catalog lists as “stocked.” The time you save in the beginning can cost double if a shipment lands in a customs limbo.
Why buy 2,6-Naphthoquinone? Some look at it for pigments, others for medical studies. Responsible buyers know why they need it, where it’ll be stored, and how to clean up after. Training matters more than access—and caring about health, safety, and local rules is part of the job, not a chore.
If you’re unsure, ask those who have ordered before—often more helpful than a faceless sales form. Building connections with suppliers improves trust and lets you stay updated on regulation changes. The chemistry community takes care of its own, especially in handling stuff that powers both great discoveries and big risks.
| Names | |
| Preferred IUPAC name | Naphthalene-2,6-dione |
| Other names |
2,6-Naphthalenedione 2,6-Dioxonaphthalene |
| Pronunciation | /ˌnæfθəʊkwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 82-41-1 |
| Beilstein Reference | 1209240 |
| ChEBI | CHEBI:16041 |
| ChEMBL | CHEMBL15804 |
| ChemSpider | 15053 |
| DrugBank | DB04353 |
| ECHA InfoCard | ECHA InfoCard: 100.003.262 |
| EC Number | 207-426-9 |
| Gmelin Reference | 72640 |
| KEGG | C04233 |
| MeSH | D009279 |
| PubChem CID | 6777 |
| RTECS number | QJ9975000 |
| UNII | YK5P3538IC |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C10H6O2 |
| Molar mass | 158.14 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | odorless |
| Density | 1.318 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.71 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 3.9 |
| Basicity (pKb) | 7.54 |
| Magnetic susceptibility (χ) | -47.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.700 |
| Viscosity | 3.63 mPa·s (at 25 °C) |
| Dipole moment | 2.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 150.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −89.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -107 kJ/mol |
| Pharmacology | |
| ATC code | D05BB10 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `O=C1C=CC=CC2=CC=CC=C12` |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| NFPA 704 (fire diamond) | 2,2,0 |
| Flash point | 138 °C |
| Autoignition temperature | 250 °C |
| Lethal dose or concentration | LD50 (oral, rat): 196 mg/kg |
| LD50 (median dose) | LD50: 196 mg/kg (rat, oral) |
| NIOSH | NA4680000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 |