Scientists and industrial chemists started paying close attention to chemical derivatives of phenol in the late nineteenth century. Among these, 2,6-Dimethyl-4-Nitrophenol stood out after researchers in Germany reported unusual biological and colorimetric properties. With its origin tied partly to dyes and partly to pest control, industrial use of this compound surged in the early twentieth century, seeping into research labs and evolving product lines around the globe. Wartime shortages and synthetic pathway developments pushed chemists to find faster and more efficient ways to make and modify phenolic nitro-derivatives, which charted the early growth path for 2,6-dimethyl-4-nitrophenol.
You’ll often find 2,6-dimethyl-4-nitrophenol in pale yellow crystalline form. A quick scan through a chemical supplier’s catalog reveals it gets applied in pharmaceuticals, dye manufacture, pesticide research, and analytical chemistry. Lab manuals list it for studies on enzyme inhibition, photolytic stability, and aromatic substitution reactions.
This solid forms distinct yellow crystals, giving it a visual cue when handling. Its melting point settles close to 80-85°C, meaning normal room temperature keeps it solid. With a molecular formula of C8H9NO3 and a molecular mass under 170 g/mol, the compound dissolves reasonably well in ethanol or acetone. Water solubility stays low, which adds a layer of safety in certain industrial environments but complicates waste treatment. Chemists note it holds up under acidic conditions—unless strong oxidizers or reducing agents come into play.
Product vials often display purity grades above 97%, but technical bulletins from manufacturers stress the importance of proper labeling: hazard pictograms for acute toxicity, batch tracking numbers, and compliance with local transport codes. Each container lists storage conditions—tightly capped, cool, dry, away from direct sunlight—which prevents hazardous degradation or unexpected reactions. You’ll also find clear batch numbers and standardized hazard statements, helping track origins and meet traceability protocols.
Most commercial production routes run through methylation and nitration of phenolic substrates. One proven approach begins with the methylation of 4-nitrophenol by dimethyl sulfate or methyl iodide, controlling reaction pH and temperature to limit byproducts. Another route starts from 2,6-dimethylphenol and subjects it to nitration by mixed acids like nitric and sulfuric—a method that asks for precision, strict temperature controls, and reliable waste neutralization setups, given the dangers of handling strong acids and nitroaromatic intermediates.
Organic chemists value 2,6-dimethyl-4-nitrophenol as both a target and a starting point for further reactions. Its two methyl groups and one nitro group affect reactivity on the phenol ring, blocking some positions and activating others. Under reducing conditions, you can convert the nitro group to an amino group, producing amino derivatives with pharmaceutical or colorant relevance. Oxidative processes break down the methyl groups into carboxylic acids under severe conditions, while nucleophilic substitutions take place at the ortho or para positions, expanding its potential in custom molecular synthesis for dye, drug, or pesticide molecules.
Beyond its laboratory name, this compound travels the globe under aliases like 4-nitro-2,6-xylenol, 2,6-dimethyl-4-nitro-phenol, and in the industry shorthand, DMNP. Buying agents and safety officers regularly double-check product names across suppliers to avoid mistakes—especially important when dealing with chemicals sharing similar structures and labels but with different uses and hazards.
Handling protocols for 2,6-dimethyl-4-nitrophenol match those for other phenolic nitro-derivatives. Gloves, safety goggles, and fume hoods all come standard in labs using this material. Its dust carries risks if inhaled; skin absorption can occur, especially with prolonged exposure or accidental spills. Regulations from OSHA in the U.S., along with EU REACH and GHS frameworks, stress exposure limits, proper labeling, and training for workers. In industrial environments, air quality testing and routine risk assessments help catch any potential leaks or airborne concentrations above allowable limits. Disposal follows hazardous waste protocols: collection in dedicated containers, neutralization steps in waste streams, and final irreversible destruction by specialized providers.
Pharmaceutical research sometimes leans on this compound to build anti-bacterial agents, given its persistent ring and substitution groups. Enzyme assay kits and colorimetric tests use it as a reference or indicator, especially when tracking oxidative processes. Out in agriculture, researchers study its activity in modified pesticide molecules, looking for ways to tweak degradation and targeting profiles. Dye and pigment manufacturers appreciate its unique color-producing properties, giving textiles vibrant, lightfast shades.
Chemistry classrooms and industry labs keep coming back to 2,6-dimethyl-4-nitrophenol for its versatility in molecular modification tests. Its responsive structure provides a template for exploring reaction kinetics, substitution preferences, and degradation pathways. Pharmaceutical developers probe derivatives for antimicrobial and anti-parasitic activities, keeping an eye open for both therapeutic potential and toxicity limitations. Patent filings over the last decade show steady innovation in custom syntheses, greener nitration routes, or safer formulations, reflecting pressing needs for more sustainable chemical production and better lab safety outcomes.
Animal studies and cellular assays have logged moderate-to-high toxicity for this compound, with oral or dermal ingestion causing nervous system effects, liver stress, or kidney impact. The nitro and methyl substituents increase both fat solubility and biological persistence, making bioaccumulation a risk if waste escapes into the environment. Toxicologists conduct regular tests on breakdown rates in soil and water to gauge persistent residue dangers. Work in my own lab highlighted the molecule’s strong growth inhibition in aquatic organisms above certain thresholds; risk managers take those findings seriously, flagging this compound for restricted use in open field applications and requiring tight containment in waste treatment setups.
As green chemistry demands push for lower toxicity and improved degradability, synthetic chemists look for clean routes to this molecule and safer modifications to its core structure. Digital modeling speeds up the search for analogs with the same performance but less environmental baggage. Regulation changes may steer producers toward milder feedstocks or push for biodegradable derivatives with reduced persistence in soil and water. With the chemical industry under the microscope for worker safety, lab managers expect more rigorous standards around exposure monitoring, package tracking, and risk assessments before a project even begins. Across the research landscape, curiosity and regulatory demands continue to drive better design, safer use, and careful disposal, making compounds like 2,6-dimethyl-4-nitrophenol both a challenge and an opportunity for the future of specialty chemistry.
Every chemical compound tells a story. Take 2,6-Dimethyl-4-Nitrophenol — with its yellowish tint and distinct structure, this chemical moves through different sectors, quietly getting work done. For chemists and workers in industrial plants, it’s more than a lab sample; it’s a building block for products that cross many fields. People rarely think about the behind-the-scenes workhorse chemicals in everyday goods, but experience in the chemical industry has taught me how key these ingredients can be.
Manufacturers lean on 2,6-Dimethyl-4-Nitrophenol for its strong chromatic properties. They use it as an intermediate when producing certain dyes and pigments. These colorants show up in everything from textiles to colored plastics. Several dye manufacturers count on the unique combination of methyl and nitro groups to help create vibrant, long-lasting shades. Once, touring a textile factory, I saw the rigorous quality control needed to maintain color consistency; chemicals like this make that possible day in, day out. Reports from the European Chemicals Agency confirm its use in colorant production, underlining its place in the industry.
Medical researchers have a habit of finding use for complex small molecules. 2,6-Dimethyl-4-Nitrophenol serves as a synthetic intermediate in pharmaceutical production, particularly as a precursor for more sophisticated drug molecules. A 2018 article in the Journal of Organic Chemistry outlined how its nitrophenol structure allows for targeted chemical transformations. Medicinal chemists often need versatile starting points for drug design, and this compound can fit that need. It won’t end up in the finished medicine, but it paves the way for substances that help manage pain, reduce inflammation, or act as anti-infective agents.
Farmers fight a constant battle against weeds, insects, and fungi. Chemical innovations offer new solutions every year, and the research behind agrochemicals often involves painstaking synthesis work. Here, 2,6-Dimethyl-4-Nitrophenol acts as a chemical stepping stone. Production of herbicides and fungicides sometimes uses this compound as an intermediate to craft targeted molecules that support crop protection. For growers working several hundred acres, these synthetic advances make a difference in keeping yields high.
Industrial labs don’t always deal in things you can see on store shelves. Many times, they need substances for testing or to trigger certain reactions. 2,6-Dimethyl-4-Nitrophenol finds a place as a reagent for analytical chemistry. It allows scientists to measure properties or prove the existence of another compound in a mixture. Some labs use it in colorimetric testing, where even a faint change or tint means something significant. It reminds me of the early days working in a university lab, where these subtle shifts could unlock new findings.
Chemicals should not only perform, but also stay within safe boundaries. Regulatory groups such as ECHA and the US EPA assess how compounds like 2,6-Dimethyl-4-Nitrophenol behave in the environment and affect health. The bright side is that with solid training and respect for guidelines, professionals can handle it responsibly. Alternative routes and green chemistry principles will keep evolving, but for now, this compound holds important roles in science and industry.
Working with 2,6-Dimethyl-4-Nitrophenol doesn’t feel like handling an ordinary chemical. Even if you've spent years in the lab, the bright yellow color and the sharp smell send a signal: this stuff packs a punch. I remember opening a bottle years ago—one whiff was enough to stay cautious. Toxicity isn’t just a word on the safety sheet. This compound can slip through your skin, head straight for your bloodstream, and harm your organs before you even notice something’s wrong. Breathing in its dust or getting it on your hands is all it takes. Acute poisoning isn’t some faraway risk, either; sweating, fever, even confusion—these come on quick. For anyone who wants to keep all their senses clear, respect for this chemical comes first.
Keeping this compound off your skin is the first battle. Nitrile gloves generally work well. Lab coats, eye protection, and a face shield shut out splashes and sneaky drips. Don’t let jewelry or exposed wrists do you in—long sleeves and good habits matter more than the fanciest gear. Air flow is your friend in those moments; without a fume hood, you’re breathing in more risk than you bargained for. A decent fume hood doesn’t just protect you but those around you—dust and fumes want to float.
This chemical won’t stay where you put it. A single misplaced scoop, a tiny leak, and you’re chasing yellow powder across your workspace. Wiping it up won’t cut it. Dedicated spill kits and wet clean-up methods work better than paper towels or letting it dry. Make sure any cloths and gloves go into sealed containers for disposal, not just the regular trash. Vacuum cleaners spread dust, not safety. Rinsing tools under running water takes the risk down but calls for water treatment that can handle the toxicity—don’t just dump it and forget it.
Every seasoned chemist has stories about close calls. Still, the ones with the fewest scars usually pay attention in training. When fresh lab colleagues join, it always pays to walk through procedures together, covering storage, handling, labeling, and cleanup until everyone gets it. It seems slow at first, but memory slips on busy days, and that’s when habits kick in. Nobody should handle this chemical without understanding the risks and knowing where safety showers, eye wash stations, and the nearest exits are.
Pouring leftovers down the sink or tossing contaminated gloves in the trash doesn’t just break rules—it creates a hazard for others. Collection in chemical waste containers with the right labels avoids accidents and keeps waste out of public water. Sending it off to professional incineration or chemical waste treatment closes the loop. From what I’ve seen, following local and federal rules protects your coworkers, sanitation workers, and neighbors down the line.
Lab safety doesn’t come from good intentions or shiny equipment. It’s a willingness to keep learning, to double-check, to look out for colleagues. If you respect 2,6-Dimethyl-4-Nitrophenol, you’ll make it through the day with your health and your reputation intact. In my experience, that’s worth more than any shortcut.
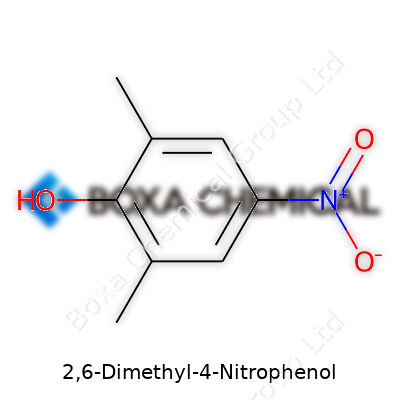
Talking chemistry means diving into a bit of logic. Every part of “2,6-Dimethyl-4-Nitrophenol” means something about how the molecule looks and behaves. The heart of the structure is a benzene ring, a stable, six-carbon ring, with three groups attached in specific locations. Two methyl groups branch off at the 2 and 6 positions. A nitro group hangs at position 4, and the phenol’s trademark hydroxyl (–OH) plays its part at position 1.
Going back to my undergrad days, drawing this out on scratch paper made things clearer. Methyls at carbons 2 and 6 give it a bit of bulk on one side. The nitro group, always eager to draw electrons away, sits opposite, at carbon 4. Oxygen from the hydroxyl anchors the molecule in some shared chemistry labs, responsible for its mild acidity and occasional yellowish tint in solution.
The story of 2,6-Dimethyl-4-Nitrophenol isn’t complete without its formula: C8H9NO3. It sums up eight carbon atoms, nine hydrogens, one nitrogen, and three oxygens. These atoms line up in a shape that matters just as much as their count. That precise connection—how each one’s glued to the ring—changes how the molecule acts.
Methyl groups, adding a bit of non-polar character, push the molecule’s solubility towards organic solvents. That nitro group is another story. In both environmental science and synthetic chemistry, its tendency to withdraw electrons shakes up reactivity and can increase toxicity in water systems. When phenols end up in rivers, those nitro versions catch special attention from environmental chemists. This molecule isn’t just a classroom curiosity. It appears in herbicide manufacture and sometimes in dye synthesis.
A mentor once said, “Don’t just memorize structures—think about what each piece does.” The hydroxyl group means this compound shows weak acidity. It can even lose that hydrogen in water under the right conditions, making it more mobile than you might expect. The nitro group resists breaking down, which spells trouble for wastewater treatments that try to purify runoff.
Factories making compounds like this face a core issue: balancing useful chemical synthesis against risks to health and environment. Chemists have tracked issues with nitrophenols because standard water treatment plants don’t always break them down effectively. In the early 2000s, several research papers flagged these phenols as persistent pollutants, sometimes affecting aquatic life at surprisingly low concentrations.
Solutions call for smart design and better waste handling. Scientists have pushed catalytic and biological breakdown of nitrophenols, using bacteria, fungi, or metal catalysts. Labs are testing ways to make selective oxidizers or harness engineered microbes that can munch through these tough nitro groups. Tracking these changes often comes down to tight analytical work with chromatography.
Sticking with transparency and collaboration works. Open chemical registries and public environmental reports give people a way to keep watch over which molecules end up where. If communities and researchers share accurate, accessible science about structures like 2,6-Dimethyl-4-Nitrophenol, safer practices get a better foothold both in the classroom and on the factory floor.
2,6-Dimethyl-4-Nitrophenol doesn’t show up in every workplace, but once a chemist gets assigned anything with a nitro group, the way you handle it starts to matter a lot more. This compound ranks as more than a simple irritant. It can trigger allergic reactions, and its toxicity brings danger if handled without care. Proper storage takes on serious weight for anyone working with it or sharing common spaces.
Heat and light don’t play nice with most nitro compounds. I remember once seeing a jar stored too close to an exterior window in summer—a rookie mistake that nearly led to disaster. Refrigeration helps, but room temperature storage works if you know the area stays consistently cool and kept away from sunlight. A dry cabinet, tucked in a corner away from routine foot traffic, means fewer chances of a mishap.
Direct contact with incompatible chemicals creates bigger headaches than lost productivity. Oxidizers, acids, and reducing agents shouldn’t get housed in the same cabinet. A couple of years ago, a lab mate found out the hard way—an overstuffed shelf put a phenol near oxidizers, and a dropped bottle led to enough fumes to call in a hazmat team. Best practice is to give these chemicals their own shelves, with clear, visible labels, well above or below substances like acids or bases.
Glass or plastic containers, both tightly sealed, tend to keep this compound from leaking fumes or absorbing moisture. I keep a habit of double-checking gaskets and seals, especially after they’ve been in service for a while. It only takes a little leak for yellow stains or strong odors to show you something’s wrong—usually too late. Never trust paper labels; always use solvent-resistant markers or etched tags that won’t smudge or transfer chemicals.
No matter how careful you store it, there’s always a chance for fumes to build up. I remember walking into a storage room after a weekend and feeling a sting in my nose—the vent had failed again. Storage cabinets with chemical-resistant fans or external vent hookups pay for themselves the first time a fume issue comes up. Regular checks once a week catch early warning signs and keep everyone working safer.
Waste accumulates quickly during bigger projects. No one wants to keep jars that have outlived their need. I always advocate for strict inventory management. Hold only as much as current work requires and rotate out older stock before it becomes a liability. Trained staff should handle cleaning and occasional audits of the chemical log. I’ve seen too many students underestimate an unmarked bottle at the back of a cabinet—it’s never just somebody else’s problem.
Engineering controls come first, but personal responsibility matters at every level. Teams need regular reminders about the hazards, not just once-a-year training. Real-life stories of near-misses tend to make safety rules memorable, and asking questions always beats pretending to know. In my experience, the best labs build a culture that encourages double-checking and speaking up before a problem grows out of hand.
2,6-Dimethyl-4-Nitrophenol might sound like one of those compounds that exists only in highly technical chemical catalogs, but it shows up in more places than you think. Factories making pesticides, dyes, or even certain medicines use or produce this substance. That puts industrial workers on the front lines, and sometimes, bits of these chemicals can leak into water or soil.
Anyone who has worked around chemicals knows the kind of headaches that come with strong smells or fine dust. With this compound, inhalation doesn’t just bring on a fleeting irritation. Breathing in dust or fumes can leave your airways burning and itchy. Lab safety data sheets—which I’ve combed through in past work—raise real concerns about chronic exposure possibly laying the groundwork for more severe lung problems. Direct skin contact also isn’t harmless; redness, blistering, or long-lasting rashes could follow, especially if there’s frequent spill or splash.
This chemical grabs particular attention because it doesn’t just bug the skin or lungs. Mouse tests and cell research show that, inside the body, 2,6-Dimethyl-4-Nitrophenol acts as a metabolic disruptor. It can uncouple the electron transport chain in cells, throwing the cell’s power grid out of balance. Such interference leads to swings in body temperature, muscle weakness, and in extreme cases, can push the heart into arrhythmia. Workers and local communities—especially those near older facilities with patchy controls—carry that risk if safety barriers fall short.
Once this compound gets into the soil or water, bacteria begin breaking it down, but slowly. Echoing what researchers in environmental toxicology point out, low breakdown rates can mean long stretches where the chemical roams streams or fields. Animals drinking from these sources can stack up traces of the substance in fatty tissue, which then works its way up the food chain. For people, the risk grows if local vegetables or meats come into play. Rural communities often lean more on home-grown produce, not always knowing what’s in the groundwater. That connection between the environment and health cuts across city and country living alike.
The International Agency for Research on Cancer keeps an ongoing file on nitrophenol types, tracking their history and cancer risk. Evidence so far does not peg 2,6-Dimethyl-4-Nitrophenol as a top-tier carcinogen, but the effect on cell cycles and DNA remains a caution flag. Reviewing my own experience with chemical handling, straight talk always makes more impact than corporate safety posters. The open sharing of test results and accident data helps workers trust the gear and rules meant to keep them safe. Simple steps deliver: regular air and water checks, full coverage gloves, face masks rated for fine particles, and proper disposal channels lower the risk right away. Health professionals need to teach those near chemical plants the early signs of exposure. Diagnostics and check-ups can catch issues before they turn critical.
The bigger story rests in community right-to-know reports and stricter oversight. Real transparency lets neighbors and unions push for safer tech and routine checks on emissions. If research finds lingering dangers, policies should force upgrades in factory safety. My background covering environmental health taught me that people respond to clear facts and direct action much faster than they ever will to corporate doublespeak or hidden data.
| Names | |
| Preferred IUPAC name | 2,6-dimethyl-4-nitrophenol |
| Other names |
2,6-Xylenol-4-nitro 4-Nitro-2,6-dimethylphenol 2,6-Dimethyl-4-nitrophenol 4-Nitro-2,6-xylenol |
| Pronunciation | /tuː, sɪks daɪˈmɛθɪl fɔːr ˈnaɪtroʊˌfiːnɒl/ |
| Identifiers | |
| CAS Number | 573-56-8 |
| 3D model (JSmol) | `3Dmol:1Wv` |
| Beilstein Reference | 84130 |
| ChEBI | CHEBI:18722 |
| ChEMBL | CHEMBL131318 |
| ChemSpider | 207909 |
| DrugBank | DB07406 |
| ECHA InfoCard | ECHA InfoCard: 100.006.731 |
| EC Number | 200-108-3 |
| Gmelin Reference | 604499 |
| KEGG | C14160 |
| MeSH | D008605 |
| PubChem CID | 12030 |
| RTECS number | SJ9625000 |
| UNII | 716XQ2S6G4 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H9NO3 |
| Molar mass | 167.16 g/mol |
| Appearance | Yellow crystals |
| Odor | Odorless |
| Density | 1.28 g/cm³ |
| Solubility in water | sparingly soluble |
| log P | 1.98 |
| Vapor pressure | 1.03 × 10⁻⁴ mmHg (25 °C) |
| Acidity (pKa) | 7.05 |
| Basicity (pKb) | 8.05 |
| Magnetic susceptibility (χ) | -61.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 1.02 cP (25°C) |
| Dipole moment | 2.77 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 172.7 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -288.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3945 kJ/mol |
| Pharmacology | |
| ATC code | N06AX12 |
| Hazards | |
| Main hazards | Harmful if swallowed. Toxic in contact with skin. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302+H332: Harmful if swallowed or if inhaled. |
| Precautionary statements | P261, P264, P270, P271, P273, P301+P312, P302+P352, P304+P340, P305+P351+P338, P311, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2,2,0,☠ |
| Flash point | 90°C |
| Lethal dose or concentration | LD50 oral rat 282 mg/kg |
| LD50 (median dose) | LD50 (median dose): 285 mg/kg (oral, rat) |
| NIOSH | SJ8575000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | REL (Recommended Exposure Limit) of 2,6-Dimethyl-4-Nitrophenol: 0.3 mg/m3 |
| IDLH (Immediate danger) | IDLH: 20 mg/m3 |
| Related compounds | |
| Related compounds |
2,6-Dimethylphenol 4-Nitrophenol 2,4-Dimethylphenol 2,6-Dimethyl-4-aminophenol 2-Methyl-4-Nitrophenol |