Curiosity drove organic chemists to explore benzoquinones more than a century ago. After 1,4-benzoquinone attracted attention for its role in oxidation-reduction reactions, scientists started tinkering with its structure. Then came the turn of 2,6-dimethoxy-1,4-benzoquinone, an engineered product that caught the eye because methoxy groups at the 2 and 6 positions change not just the look of the molecule, but the way it behaves. Developments in the late 20th century made it easier to isolate and purify this specific derivative, unlocking its use in chemical research and, interestingly, sparking new routes in pesticide and plant science studies. The path to today came littered with both laboratory surprise and persistence at the bench, as researchers pushed on for molecules that could serve purposes beyond what parent benzoquinone allowed.
The compound holds a discreet but valuable spot in both laboratory research and potential industrial applications. Most pure samples appear as yellow crystalline solids, and their unique properties find use in electrochemistry, organic synthesis, and even plant defense research. Scientists prize it for its strong oxidative capacity, which makes it a convenient electron transfer agent in a range of biochemical and physical processes. Wherever there's a need for a compact redox-active molecule that brings specific reactivity, 2,6-dimethoxy-1,4-benzoquinone enters the conversation.
In my experience, 2,6-dimethoxy-1,4-benzoquinone stands out because of its crystallinity and clear, bright color, which hints at purity when first drawn from the bottle. Its molecular formula is C8H8O4, and it comes with a predictable melting point usually near 138°C. The chemical demonstrates solubility in a range of organic solvents, from acetone to ether, letting researchers work with it in flexible settings. Not only does this compound display classic benzoquinone characteristics, it brings tweaked redox potentials—making it a strong pick for reactions dependent on selective electron flow. Methoxy substituents shield the aromatic core and drive specific reactivity in both synthesis and analysis.
No one wants confusion in the stockroom, so labeling on commercial 2,6-dimethoxy-1,4-benzoquinone bottles gets straight to the point: chemical name, molecular weight, purity (often 98%+ by HPLC or GC), and recommended storage away from light and moisture, to preserve both color and power. Manufacturing lots come with batch-specific certificates of analysis, so buyers track what they're actually working with, which makes reproducibility less of a gamble. Lab users can expect details on appearance, melting point, and, for research purposes, spectral data for IR and NMR, not just a chemical name and a warning sign.
Synthesis typically starts with 2,6-dimethoxyaniline, leading into a controlled oxidation process. Researchers favor agents like silver oxide or chemical oxidants such as ceric ammonium nitrate, both of which facilitate the creation of the desired quinone structure without causing unwanted over-oxidation or decomposition. The process demands close attention to reaction time, temperature, and intermediate processing steps, including careful filtration and crystallization—because impurities or trace byproducts can ruin downstream experiments. In my work, I learned quickly that even small deviations in oxidant strength or timing turn a bright yellow batch pale, a clear sign you need to start over.
Once in hand, 2,6-dimethoxy-1,4-benzoquinone lends itself to all sorts of creative transformations. It readily participates in nucleophilic aromatic substitutions, letting chemists modify positions neighboring the benzoquinone core. Its reactivity as an electron acceptor opens doors in radical-based synthesis, too. I've used it to generate semiquinone intermediates that serve as probes for mechanistic studies. Reduction brings it back to the corresponding hydroquinone, a reversible process that anchors its use in redox chemistry. Besides modifications, the molecule can act as a starting point for building more complex derivatives used in pharmaceuticals and agrochemicals.
You might come across this compound under other names: 2,6-dimethoxy-p-benzoquinone, DMBQ, or by its registry number. Suppliers sometimes use trade names for convenience, but the core identification centers around the dimethoxy and benzoquinone tags. For those hunting through catalogs, knowing alternate names keeps searching efficient, especially when jumping between suppliers or institutional databases.
This compound doesn’t fall into the toxic category like some industrial benzoquinones, but proper handling matters. Prolonged exposure to dust or powder can irritate the skin, eyes, and respiratory system. Wearing gloves and working inside a fume hood have always been my default moves. Material safety data sheets stress limited contact and solid ventilation, reminding us that the sense of smell shouldn’t be the first line of detection for leaks. Safe storage relies on bottling the compound away from heat, open flames, and oxidizing agents. Laboratories working with larger amounts should keep spill kits and neutralizing agents on hand for emergencies.
The research reach stretches from organic synthesis—where it acts as a strong oxidant or a building block for more elaborate molecules—to plant science, where biologists use it to study signal transduction and stress responses in crops. Plant physiologists observe its role in simulating oxidative stress, which helps them tweak breeding and develop hardier agricultural varieties. It doesn’t stop at biology. Physicists and electronic engineers harness the redox cycling of DMBQ for designing sensors, batteries, and specialized catalysts. Its stable structure also makes it a tool for calibrating equipment or validating new analytical methods, bridging organic chemistry, materials engineering, and biotechnology.
There’s always a push for greener, safer, and more efficient chemical processes, and 2,6-dimethoxy-1,4-benzoquinone sits right in the middle of these discussions. Researchers continue to study its effects on plant cell respiration and signaling, hoping to draw links between artificial stressors and natural plant defense mechanisms. Synthetic chemists work to invent faster, one-pot methods for its generation, using sustainable oxidants and less energy-intensive conditions. These advancements lead to more consistent quality, batch to batch, and reduce the environmental impact of chemical production—a win for both science and the planet. Keeping up with published literature, I’ve noticed a surge in interest for its role in enzyme-mimetic catalysis, hinting at untapped industrial value.
No chemical story matters if it ignores long-term health and environmental risks. Toxicologists have tested DMBQ against standard assay batteries. At typical research concentrations, acute toxicity stays low, but like most quinones, high doses or chronic exposure trigger oxidative stress in animal and plant cells, resulting in cytotoxic effects. Aquatic toxicity has received increasing scrutiny because benzoquinones, in general, can disrupt waterborne microbial communities and invertebrate development. Lab results suggest that with proper handling and disposal, risk remains low, but scaling up activities or accidental release would demand careful monitoring and next-step studies. Toxicity to plants, especially, holds both positive and negative implications—crop protection at one end, but potential for environmental imbalance at the other.
Opportunities open up as more researchers recognize the specific control DMBQ offers in electronic, catalytic, and biological systems. Interest in organic electronics—especially flexible sensors and thin-film batteries—has driven new studies into the durability and charge transport behavior of DMBQ-based materials. Advances in agricultural biotechnology might lead to its use in seed treatments or as a template for next-generation fungicides, though any move to the field hinges on demonstrating both safety and effectiveness outside the lab. I see more focus on sustainable production, where chemical manufacturers experiment with bio-based oxidants and waste minimization, linking cost-saving with global responsibility. If those in the community keep safety, innovation, and collaboration at the forefront, DMBQ’s legacy will be less about what it started as, and more about what creative researchers help it become.
2,6-Dimethoxy-1,4-benzoquinone shows up in more chemistry journals than most would expect. It’s a yellow crystalline solid, a classic benzoquinone, but what really grabs attention is its two methoxy groups at specific spots on the ring. The presence of those groups changes how the molecule behaves — and that’s exactly what makes it useful in multiple labs and products.
Researchers have spent years looking for antioxidants that can hold their own in harsh environments. This compound pops up as a robust electron acceptor in redox chemistry. For anyone who has tried to develop dye-sensitized solar cells or fine-tune the electron flow in bioelectronic devices, its stable redox properties offer a dependable option. In plant physiology studies, scientists often use 2,6-dimethoxy-1,4-benzoquinone to understand how plants respond to stress. Its ability to mimic what naturally occurs in plant cell signaling comes in handy in these experiments.
Take a look at biosensor technologies. Building a sensor that can read subtle biological signals takes molecules that actively participate in electron transfers. This benzoquinone stands out for its predictable behavior in redox cycles, often serving as a model compound. Teams working on medical diagnostics benefit from the easy way it facilitates electron jumps between proteins and sensor electrodes, raising the sensitivity of the devices without a fuss.
Plant cell culture researchers recognize this chemical for its influence on cellular respiration and stress responses. Certain studies investigate its effect on root development or its role in stimulating secondary metabolite production. Plant tissue culture faces plenty of challenges, from contamination to unwanted cell death. Adding 2,6-dimethoxy-1,4-benzoquinone sometimes helps maintain healthy cultures, opening more doors for sustainable agriculture, especially for crops that struggle under modern farming pressures.
Some medical researchers pursue it as a path toward understanding or fighting cancer. A few studies highlight how it might interfere with tumor cell processes, sometimes slowing their growth or making them more responsive to treatments. In the world of microbiology, labs use it to investigate oxidative stress in bacteria and fungi. As infections become more resistant worldwide, that knowledge might lead to new antibiotics or safer agricultural fungicides.
Every chemical carries responsibility. Safety data for 2,6-dimethoxy-1,4-benzoquinone points to risks with skin or eye contact, so gloves, goggles, and careful handling really matter. Disposal needs careful attention, too, following legal and environmental rules. Manufacturers now face the question of sustainable production, keeping environmental impact low, and reducing hazardous waste.
Access to this compound supports not just academic research, but solutions to real-world problems: better crop yields, improved diagnostic tools, and more effective therapies. As with any tool, purpose and context shape the real benefit. Collaboration between chemists, plant biologists, clinicians, and regulators offers the best chance for this compound to live up to its potential—grounded in evidence and built on responsible stewardship.
2,6-Dimethoxy-1,4-Benzoquinone carries the chemical formula C8H8O4. For chemists, this tells a simple story: the molecule has eight carbon atoms, eight hydrogens, and four oxygens. The molar mass sits at 168.15 g/mol. That number might sound dull, but checking it for accuracy means checking the integrity of thousands of lab results that use this compound.
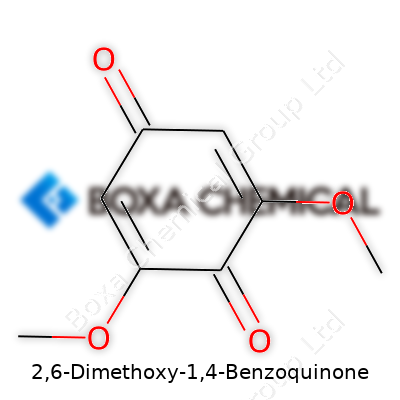
With two methoxy groups hanging from a benzoquinone ring, 2,6-Dimethoxy-1,4-Benzoquinone stands out even among other benzoquinones. The structure pushes electrons around in a pattern that draws in scientists interested in redox chemistry. People in the field will recognize its shape from aromatic chemistry texts where quinones play a role in electron transfer. These transfers show up everywhere — from dying fabrics to early studies on cellular respiration. I’ve seen people build experiments on electron acceptors, and this compound seems to pop up more often than the typical organic molecule.
Interest isn’t just academic. This compound holds value in biological and environmental studies. Researchers used similar structures to probe the ways plants and fungi communicate or defend themselves. Quinones often act as chemical signals or toxins in nature. For instance, some wood-decomposing fungi depend on benzoquinones to break down tough lignin structures, helping soil ecosystems recycle dead plant matter. When labs look for models to study this kind of chemistry, 2,6-Dimethoxy-1,4-Benzoquinone fits the bill.
Beyond nature, synthetic chemists like having benchmarks for redox reactions. Controlling electron flow underpins a lot of modern technology. Scientists want compounds that behave reliably in electron exchanges, and compounds like this aren’t rare in academic collections because their reactions give clear, reproducible results. It showcases chemical behavior many undergraduates see for the first time in labs.
Accuracy with data points like formula and molecular weight matters for more than reporting. One decimal in the wrong spot can waste hours of work downstream. Analytical labs doublecheck these numbers before mixing the compound with anything. I’ve learned to trust but verify every molecular weight I use. Even printed catalogs sometimes carry small mistakes, and batch-to-batch purity can throw off numbers that should line up.
There’s no shortage of misinformation around chemical identities. I’ve fielded questions from new researchers who rely on secondary sources or free databases and get derailed by typos. The best solution remains to trace information back to published, peer-reviewed material or databases known for reliability. Physical copies of organic chemistry handbooks don’t gather much dust, because having an authoritative reference saves more time than it costs to doublecheck.
I see educators put a lot of focus on the basics — formula, weight, and safe handling. This discipline pays off again and again, because solid chemistry always starts with trustworthy numbers.
Many chemicals lose their punch or, worse, turn dangerous when left out or handled the wrong way. Over the years, I’ve seen clean, shelf-stable compounds ruined by a bit too much humidity or a careless lid left loose. 2,6-Dimethoxy-1,4-benzoquinone isn’t any different. In research labs and chemical supply closets, this compound needs a good spot, set at the right temperature, and well away from things that might set off a reaction.
Overexposure to light can shift the composition of quinones before you realize it. Strong sunlight or even bright lab lights speed up degradation. For this reason, I keep it in a tightly sealed amber bottle. I make sure to put the bottle in a locked fridge or, at the very least, a cool, dark cabinet that's not opened and closed all day. The target spot tends to hover around 2-8°C, which slows down any unwanted reactions.
On my own shelves, sticking to low temperatures made a real difference. Compounds that spent a week out warm and exposed often ended up clumpy or discolored. You need only one bad batch to learn that point for good.
Quinones, like this one, do not get along with moisture or oxygen. Even a tiny amount of water vapor shortens shelf-life and can encourage breakdown. I use silica gel packs in every storage container—the little blue beads turn pink when they pick up moisture, signaling it’s time to swap them out. Oxygen is another unseen enemy; after each use, I squeeze out the air before sealing bottles tight.
A lab I worked in kept running into problems until we added a glove box filled with inert nitrogen for opening and closing reactive chemicals. For those without expensive setups, using freshly dried bottles and working quickly helps keep air out. These steps pay off in reduced waste and less risk.
Clear labeling stops mistakes before they start. I find handwritten dates and user initials work. No one wants a mystery bottle with a faded label and a silent history. Regular inventory checks flush out old material and flag any problems—such as crystals forming or color shifts—before they get worse.
Chemical hygiene saves money, time, and sometimes skin. Many accidents come down to someone storing the wrong thing next to the wrong chemical or forgetting what’s in a container entirely. An extra five minutes labeling and double-checking pays back all year.
No storage method lasts forever. Expired or degraded 2,6-dimethoxy-1,4-benzoquinone must go in a proper hazardous waste stream. I always follow university or local regulations for chemical disposal—pouring anything down the drain risks fines and environmental headaches. Good gloves, goggles, and fume hoods are non-negotiable for safe handling.
Those who treat benzoquinones as casual bench ingredients soon face ruined experiments or worse. Experience teaches that a cool, dry, and dark spot is best. Consistent labeling, airtight seals, and minimizing oxygen and water stretch the shelf life and support safety. Keeping up with these habits feels tedious some days, but disaster only strikes once to make a believer out of anybody.
2,6-Dimethoxy-1,4-benzoquinone comes from a class of compounds known as quinones. You’ll usually find it in research labs, sometimes surfacing in studies of plant biochemistry or as a building block for dyes and pharmaceuticals. The structure feels pretty basic—a ring with two methoxy groups stuck onto the quinone skeleton—but the effects of a molecule like this don’t always stay in the safe zone just because it appears simple.
To understand toxicity, you want specifics. Research points out that many benzoquinone derivatives, especially the unsubstituted forms, can irritate skin and eyes. They can cause inflammation or allergic reactions for lab workers handling the powders without gloves. If this compound lands inside the eye, it usually stings immediately, so good lab goggles turn essential for direct work.
Studies published in toxicology journals show that modifications, like adding methoxy groups, sometimes cut back the intensity of these reactions but don’t wipe them out. 2,6-Dimethoxy-1,4-benzoquinone still belongs to a chemical family notorious for forming reactive oxygen species, which can mess with living cells by promoting oxidative stress. In cell models, this leads to cell death or damage in high enough concentrations.
Animal studies give only limited answers. Few scientists test this molecule directly in full animal systems because most available data focuses on its close relatives. Even so, quinones in general disrupt biological function by interacting with proteins and DNA. That kind of reactivity has a double-edged blade; researchers look for it in cancer drug concepts but run for the fire extinguisher if these substances spill during experiments.
Back in my postgrad research days, nobody let us near quinones without running through a full safety briefing. The potential for skin rashes lingered with every bottle. We kept decent fume hoods going to capture the dust. Public safety data sheets highlight the importance of ventilation, gloves, and even face shields now, because breathing powdered quinone down the nose makes the lungs cough and the eyes water in no time.
The environmental impact also needs some attention. Benzoquinones break down over time but tend to linger in water supplies if they escape down laboratory drains. Persistent exposure to fish or aquatic insects leads to lethal outcomes, since these compounds interrupt the usual balance of life by gumming up cellular machinery. Universities stress proper disposal—chemical waste bins, not sinks.
Good habits make the biggest difference. Never overlook the value of personal protective equipment—scientific gloves, splash-proof goggles, and tightly sealed containers keep accidents rare. It helps to keep a clear plan in the lab for cleaning up spills and making quick decisions if someone touches or inhales the stuff by mistake. For broader safety, labs should keep training updated and encourage users to review the latest literature. Students new to chemicals like 2,6-dimethoxy-1,4-benzoquinone need easy-to-understand briefings, not just dense protocol sheets.
To keep communities safer, look at ways to substitute less reactive chemicals in routine experiments. Sometimes, even a slight tweak in research routines reduces the need for quinones, cutting down exposure. And don’t forget effective waste collection systems—solid containers, clear labels, and scheduled pickups shut down accidental contamination of air and water.
2,6-Dimethoxy-1,4-Benzoquinone—often abbreviated as DMBQ—turns up in places most folks never think about. For those who spend time in a chemistry lab or work in sectors that innovate around plants and medicine, DMBQ isn’t just a set of odd syllables; it’s a building block. I’ve watched research crews add small vials of this compound to flasks, and the impact can ripple out in surprising directions.
DMBQ comes up again and again in plant biochemistry research. Scientists look to it as a reference point for redox reactions in living cells, since it can accept and donate electrons. The way DMBQ interacts with enzymes in plant cells gives clues about how plants handle stress, fight off infection, or power their growth. Some teams use it as a stand-in for natural plant compounds that are hard to purify, giving them a shortcut for studying how certain molecules affect cellular machinery.
Outside the test tube, I’ve seen collaborations between chemists and pharmacologists that use DMBQ during early drug screening. Researchers know that compounds with quinone groups, like DMBQ, can act as starting templates for drugs that kill bacteria or cancer cells. There’s a growing push for new antibiotics and anti-cancer agents, and DMBQ’s basic structure makes it a candidate for modification. Labs sometimes start with DMBQ and swap out parts of the molecule to see if they get stronger or safer biologically active chemicals.
Anyone who reads food labels has seen the word “antioxidant,” but testing these claims needs reliable methods. DMBQ helps here, too. It usually gets added to systems designed to measure antioxidant capacity because its redox potential sits in a range similar to the natural oxidants found in fruits and vegetables. When DMBQ reacts in these systems, the results tell researchers how strong an antioxidant really is. Companies that make dietary supplements depend on these numbers to market products and pass regulatory checks.
You’d think this chemical would stay locked in a lab, but DMBQ finds its way into the world of sensors and batteries. Its ability to rapidly swap electrons makes it helpful in the development of electrodes for electrochemical sensors. Some diagnostics use sensors built with DMBQ as part of their detection system, especially for tracking glucose or environmental toxins. Researchers create test strips or chips where DMBQ acts as a mediator, shuttling electrons between a biological sample and electronic measuring device. Improvements in accuracy or lower cost usually start with these kinds of tweaks at the molecule level.
While research opens doors, safety does matter. DMBQ doesn’t belong in food or cosmetics, and handling requires solid training. Its reactivity serves science but also means it can harm cells if misused. I’ve seen guidelines posted in labs, urging chemists to wear gloves and keep exposure time short. For broader use, additional toxicity data would help, giving regulators and innovators a way to balance scientific promise with health and environmental safeguards.
Progress depends on three things—open data, smarter experimental design, and patient collaboration. Researchers need access to reproducible results, so journals and companies benefit from sharing both positive and negative findings around DMBQ. Universities and startups can work side by side, aiming for safer molecules and better gadgetry. Students want to see chemistry connect to real-world impact, and in that sense, DMBQ isn’t just a name in a catalog—it’s a stepping stone toward technologies that touch health, food, and beyond.
| Names | |
| Preferred IUPAC name | 2,6-dimethoxycyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /tuː sɪks daɪˌmɛθ.ˈɒk.si waɪn ˌbɛn.zəʊ.kwɪˈnəʊn/ |
| Identifiers | |
| CAS Number | 620-97-7 |
| Beilstein Reference | 1000391 |
| ChEBI | CHEBI:4059 |
| ChEMBL | CHEMBL236579 |
| ChemSpider | 160979 |
| DrugBank | DB04199 |
| ECHA InfoCard | 04d1b2af-2f3a-4ba6-aa37-0e9b394fa87f |
| EC Number | EC 208-341-8 |
| Gmelin Reference | 82253 |
| KEGG | C06512 |
| MeSH | D016692 |
| PubChem CID | 70106 |
| RTECS number | DE1225000 |
| UNII | 8YK32D21JS |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H8O4 |
| Molar mass | 198.16 g/mol |
| Appearance | Yellow solid |
| Odor | Odorless |
| Density | 1.32 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 0.37 |
| Vapor pressure | 1.5 x 10^-3 mmHg (25°C) |
| Acidity (pKa) | 3.20 |
| Basicity (pKb) | 9.01 |
| Magnetic susceptibility (χ) | -46.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.603 |
| Viscosity | 2.38 mPa·s (at 25 °C) |
| Dipole moment | 3.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 207.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -275.2 kJ mol^-1 |
| Std enthalpy of combustion (ΔcH⦵298) | -1099 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 138°C |
| Autoignition temperature | 185 °C |
| Lethal dose or concentration | Rat oral LD50 200 mg/kg |
| LD50 (median dose) | LD50 (oral, rat) 200 mg/kg |
| NIOSH | DA2975000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |