For decades, synthetic antioxidants reshaped materials science and chemical engineering, and 2,6-Di-Tert-Butyl-4-Ethylphenol (DTBEP) made its appearance right as polymer and fuel industries began searching for more effective ways to combat degradation. As oil derivatives started dominating manufacturing and processing in the last century, scientists noticed common phenolic stabilizers couldn't quite handle newer, more volatile blends. The introduction of tert-butyl groups onto phenol rings denoted a shift in synthetic methods, steering development away from simple alkylation processes and toward specialized, sterically hindered substitutes like DTBEP. Formulators in the 1970s and '80s, looking for antioxidants that lasted longer at high temperatures, found this compound reliable after observing oxidation challenges in the first automotive plastics and synthetic lubricants.
DTBEP falls into the family of hindered phenols. Here’s a molecule designed for heat stability, resistant to breakdown, shielding polymers and fuels during storage and operation. This chemical traces its influence wherever oxidative wear can shorten product life—blending oil, thermoplastics, synthetic rubber, adhesives. Suppliers bottle it in fine, free-flowing powder, often with only faint odor thanks to its structure. Batch-to-batch consistency matters here, since clients buying lots for plastics don’t want variable melt flow or color contamination. Global trade catalogs label DTBEP as an essential non-staining antioxidant, which appeals to engineers balancing function with appearances in final goods.
DTBEP appears as a pale yellow crystalline solid at room temperature, melting around 45–49°C, and boiling upwards of 320°C under reduced pressure—attributes influenced directly by those bulky tert-butyl groups framing the phenol ring. In the lab, it resists water, floats in most organic solvents, and doesn't dissolve quickly; this low solubility in water makes sense, as it was never meant to leave the matrix it’s protecting. The high molecular weight, roughly 262, puts it out of the volatilization range for most consumer applications, meaning it won’t easily evaporate from plastic or oil blends. Chemically, it remains neutral in most environments, holding up under routine light and air exposure.
Manufacturers list purity of DTBEP typically above 99%, checked using gas chromatography, with residual solvents and heavy metals well below regulated levels. Packaging standards matter for bulk buyers; woven bags or metal drums lined with polyethylene prevent outside contamination and accidental exposure. Labels highlight CAS numbers for traceability, batch codes, manufacturing date, and shelf life, giving purchasers ways to audit each delivery. Regulatory notes often include compliance with regional guidelines for chemical import, export, and use—such as REACH, TSCA, and other frameworks.
Chemists synthesize DTBEP from the alkylation of ethylphenols with tert-butyl alcohol, commonly under acidic catalysis. Labs scale this up using para-ethylphenol mixed with excess tert-butyl alcohol and a strong acid like sulfuric acid, followed by an extraction process to remove undesired by-products. At plant level, continuous reactors allow precise control of temperature, flow rates, and mixing, critical for limiting trace impurities. Purification involves crystallization and filtration stages, since only low levels of residual acid or unreacted starting material are tolerated in technical and food-contact applications.
Within DTBEP’s crowded aromatic ring, those tert-butyl and ethyl groups crowd out most reaction partners. Still, chemists look for ways to tweak its backbone for experimental blends. Functionalization on the para-position—already blocked by ethyl—doesn’t come easy. The molecule’s antioxidant capacity owes itself to the phenolic OH group, which donates hydrogen and captures radicals, forming a stabilized phenoxy radical lasting longer than simpler phenols. Direct derivatization hardly ever outperforms the original structure, so most modifications involve blending DTBEP with synergists like phosphites, thioethers, or amines to dial up antioxidant effectiveness for demanding process conditions.
DTBEP answers to many aliases: Antioxidant 24, 2,6-Bis(1,1-dimethylethyl)-4-ethylphenol, or sometimes just BHT-Ethyl. By comparing catalog entries from Asian, European, and American suppliers, one begins to spot minor naming quirks but identical CAS assignment. Some manufacturers slap on proprietary codes or trade names, especially for ready-made blends designed for plastics or oil additivation, leading to some confusion for buyers who must check chemical identities with each purchase order.
Anyone working with DTBEP in an industrial mixing area or a quality control lab needs clear guidelines. Skin and eye contact can cause irritation; chronic inhalation or ingestion—by accident or poor practice—leads to subtle toxicity symptoms observed in animal testing such as enzyme changes or liver effects at very high doses. Material Safety Data Sheets flag basic PPE requirements: gloves, goggles, dust masks, and good ventilation. Facilities install spill trays and dust extractors in handling zones because fine powder can disperse and settle quickly. Waste management involves off-site disposal with specialty contractors following hazardous waste regulations. Factory SOPs stress regular equipment cleaning and leak checks to prevent contamination of finished goods.
DTBEP’s star role shines in stabilizing polymers used in automotive interiors, electrical casing, appliances, synthetic rubber gaskets, and high-value industrial belts. Its utility stretches further into lubricant blends, fuel additives, and adhesives—especially where regulations limit more volatile or staining stabilizers. Over time, I’ve watched engineers choose DTBEP not just for its raw effectiveness, but for how predictably it performs against thermal aging, color changes, and embrittlement—a recurring problem for outdoor and high-friction applications. Both large processors and niche compounders choose it to extend shelf life and cut maintenance costs. Specialty resins for microelectronics or medical tubing sometimes depend on DTBEP for stability inside complex formulations.
Recent years brought a wave of studies focused on how antioxidants like DTBEP interact with novel bioplastics, biodegradable polylactic acid (PLA), or next-generation rubber mixes. Researchers investigate pairing it with other stabilizers to cut down on additive build-up, yellowing, or migration during heat cycling. Projects examine long-term effects in recycled content polymers, given the global push for sustainability and the need for antioxidants that don't interfere with reprocessing. Lab teams use high-throughput screening to test DTBEP under simulated use conditions—cycling humidity, heat, and UV—to map degradation and identify breakdown thresholds. Universities and corporate R&D centers dig into alternative synthetic routes for the molecule, responding both to regulatory pressure and steady demand growth across Asia and emerging economies.
Animal studies show DTBEP delivers low acute toxicity, but like its relatives, it doesn’t pass through biological systems completely untouched. Oral exposures in lab animals provoke mild effects only at levels far above practical workplace exposure, but the slow metabolism and bioaccumulation possibilities drive further research. In vitro tests have not linked it strongly to genotoxic effects, though regulatory bodies encourage tracking long-term exposure risks, especially in recycled consumer products that may leach additives under humidity or acidic conditions. No clear evidence suggests immediate health threats to the public at standard use concentrations in polymers or fuels, but manufacturers run routine monitoring and exposure assessments throughout the processing chain.
New market directions keep expanding the demand for robust antioxidants like DTBEP, including in energy storage, green materials, and high-stress industrial composites. Increased regulatory scrutiny on additive leaching and toxicity means companies focus not just on performance, but also on environmental and human health profiles. Labs look for ways to integrate DTBEP in blends that work for recycling, recover more easily, or biodegrade with less trace residue. Newer methods using greener chemistry for manufacturing, and life-cycle studies for approved uses, will likely influence which applications DTBEP enters next. As sustainability becomes more than a buzzword, the road ahead demands innovators balance proven chemistry like DTBEP with evolving standards and public scrutiny.
2,6-Di-Tert-Butyl-4-Ethylphenol might seem like a tongue-twister, but it fills a gap in keeping our daily-use goods lasting much longer. Used mainly as an antioxidant, this compound steps up to prevent a process called oxidation, which ruins plastics, rubbers, and fuels. It slows down the breakdown that heat, light, and air can spark in many modern materials.
I’ve worked with plastics at a packaging plant. We fought material failures and color changes in products left too long on store shelves. Every time a batch went bad, the cost hit not just the plant but shops relying on our delivery. Most folks don’t think about it, but imagine buying a garden hose only for it to crack under a summer sun after just one season. The same goes for fuel sitting inside car tanks or machinery. Without these stabilizers, manufacturing costs shoot up, and so do prices for customers.
Gasoline and diesel contain mixtures that don’t get along well with oxygen and metal tanks. They start to form gum or sticky deposits that clog engines after only a few weeks or months. 2,6-Di-Tert-Butyl-4-Ethylphenol finds use in stopping this gumming process, letting engines run smoothly. The stability added to fuel also keeps vehicles and generators dependable after long months of storage.
Think about food containers, wire coatings, or even playground equipment. Sunlight and exposure to air slowly attack the plastic, making it brittle or yellow. Antioxidants like 2,6-Di-Tert-Butyl-4-Ethylphenol offer a line of defense, holding the original strength and color longer, which means less waste and fewer replacements. Less frequent failure also helps keep things out of landfills, cutting environmental impact.
People worry about what goes into plastics, especially for food packaging. Studies and toxicological assessments have shaped regulations around how much of these chemicals can be used. Extensive reviews help keep risks in check so the public can benefit from safer, longer-lasting products. It’s important manufacturers follow those guidelines, as trust drops sharply with any health scare or product recall. Sperling et al. (2023) noted how regulatory agencies in the EU and US regularly look at additives like 2,6-Di-Tert-Butyl-4-Ethylphenol for any sign of risk, reflecting how science and safety move together.
Chemists keep trying to find antioxidants that last longer and come from safer sources. Sustainable options—sometimes derived from plants—are being developed. Recycling plants now pay close attention to how old antioxidants in discarded plastics react when processed together. This means future innovation should bring additives that both protect materials and balance health or environmental safety. Social pressure and consumer habits add another push towards ‘greener’ additives beyond synthetic staples like 2,6-Di-Tert-Butyl-4-Ethylphenol.
Without this obscure-sounding chemical and its relatives, everyday goods wouldn’t give the same value. Food might go bad faster, car parts would break sooner, and shopping bills would jump. Whether through better regulation or green chemistry, these antioxidants help bridge the gap between convenience and durability.
Ask anyone who’s spent time in an industrial lab or a university stockroom about 2,6-Di-Tert-Butyl-4-Ethylphenol—DTBEP really is a mouthful and is easier to call what most chemists do: an antioxidant additive. You’ll find it in plastics, rubber, and even lube oils. That stuff helps keep materials from falling apart too soon, blocking some of the damage caused by heat, air, and sunlight. People working with 2,6-Di-Tert-Butyl-4-Ethylphenol want to know if it’s safe to use. I’ve handled plenty of phenols in my time, and respect for the risk comes from how easy it is to take safety for granted when the hazards aren’t obvious.
The chemical name sounds intimidating. The hazards are more about slow and sneaky effects than instant disaster. Safety datasheets list skin and eye irritation—nothing out of the ordinary for phenol compounds. A splash or spill might not burn right through a glove, but a day of careless exposure can leave your skin red and cracked. The vapor doesn’t swing the punch of something volatile like acetone, but there’s no need to be breathing any extra organics. Most of us remember the stories of old-timers who ignored PPE, only to discover a rash or a cough that lingered for weeks.
Written evidence in the scientific journals points to low acute toxicity by mouth or skin, with LD50 values for rats in the range of 1,600 mg/kg. In one workplace incident I witnessed, a mishap left a small amount on a table; it didn’t create panic, but the spill still meant scrubbing down gloves and surfaces. Immediate cleanup was easy. But let a mess sit, and there comes a sticky residue that can transfer and build up. Chronic exposure might nudge your liver or kidneys into trouble, so regular careless handling isn’t an option. This isn’t just theoretical talk either—phenol derivatives are known for their persistent bioaccumulation, with long-term effects often underestimated.
Tipping waste down the drain isn’t smart. I’ve seen how easier clean-up methods can tempt people to skip steps. That shortcut risks water sources. Persistence in the environment sticks around—phenolic compounds don’t break down quickly. Wildlife faces toxic hits, with aquatic organisms most at risk. The real trouble starts when small mistakes add up, hitting the same patch of soil or creek over months.
Direct handling means goggles, gloves, and good air. I learned the hard way to swap out torn gloves—cheap nitrile breaks fast with some organics. Good ventilation takes care of any lingering fumes. Breaking bad habits comes from training and experience. Supervisors play a huge role, but so does peer pressure. Sharing stories about what happens when mistakes pile up makes the safety rules stick better than any sign on the wall.
Regular audits and spill drills keep teams ready and honest. Waste stays sealed, logged, then shipped for proper disposal. Chemistry isn’t about courage—it’s about practicality. Getting the job done right means keeping yourself and your workspace healthy. That makes for safer coworkers, cleaner systems, and a lighter impact on everything downstream.
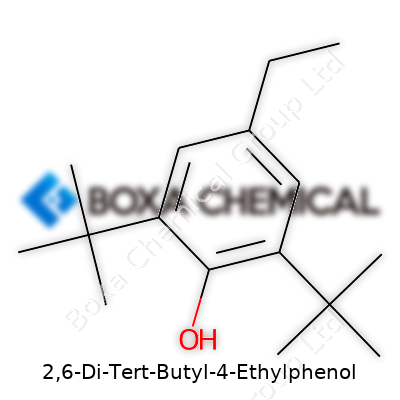
2,6-Di-Tert-Butyl-4-Ethylphenol, more familiar to chemists as DBEP, has a name that sounds like a tongue-twister but a structure that sparks genuine interest, especially for anyone fascinated by the chemistry of antioxidants. Its structure, at the core, centers on a phenol ring—a classic six-carbon aromatic ring with an -OH group attached. That’s a pattern you see in many antioxidants, especially those that need to stand up to oxygen and heat in tough industrial settings.
On two spots, right next to the hydroxyl group (the 2 and 6 positions of the ring), bulky tert-butyl groups stick out. These tert-butyl arms are big and awkward—like elbows poking out at a crowded theater—which keep other molecules from getting too close to the reactive parts of the phenol ring. One more piece—the ethyl group—sits at the 4 position, rounding out the flavor of the molecule. So in shorthand, you can picture DBEP as a phenol ring with two tert-butyls crouching at the top and an ethyl group anchoring the bottom.
The addition of tert-butyl groups isn’t just for show. These big, branching groups offer the molecule stability, so it does a better job as an antioxidant. In regular day-to-day chemistry, phenol can donate a hydrogen atom from its -OH group to stop free radicals in their tracks, breaking the chain reactions behind unwanted oxidation. Those tert-butyl groups protect the molecule, slowing down how quickly oxygen or other free radicals can attack the ring. That keeps the antioxidant working longer, which matters if you’re protecting rubber in car tires or the oil in a frying pan.
The ethyl group at the 4 position doesn’t just hang there, either. It adds a touch more stability and changes how the molecule dissolves in different mixtures. These tweaks shape how companies use DBEP—not just as a science project but as a real ingredient in products you might use without thinking. For instance, the food and beverage industry cares about shelf life. Synthetic antioxidants like DBEP help lengthen that, cutting down on spoilage. In plastics and rubber, this chemical keeps products from cracking or falling apart when exposed to sunlight and air.
Compounds with bulky alkyl groups, like DBEP, tend to stick around in the environment longer than simpler phenols. There’s always a tradeoff in chemistry between making a chemical durable enough to work and making sure it doesn’t stick around where it’s not wanted. As someone who has seen factory work up close, seeing how regulatory teams check run-offs and monitor waste, I know decisions about these chemicals carry real weight. Food safety labs keep a close eye on how much of these antioxidants can end up in edible oils, using standards set by authorities like the FDA and EFSA. Factories invest in capturing and breaking down waste streams when using DBEP or similar molecules.
Many researchers keep searching for ways to match the performance of DBEP with greener ingredients, hoping to strike a better balance between performance, cost, and long-term safety. Some teams study blends of natural antioxidants, or keep tweaking those bulky groups to break down more easily after they do their job. Progress comes slowly, but constant pressure from both scientists and communities keeps this work moving.
Clean chemistry isn’t a pipe dream. It starts by making each step—from lab design to large-scale use—a little better. Engineers work on reactors that use less energy and make less waste. Chemists run tests to find out if slightly changing the bulk of the groups can help the molecule break apart faster in soil or water. Many companies push for fuller transparency, labeling not just what’s in a product but why it’s there.
This sort of progress usually feels more like patching a roof than building a perfect new house in one go. But even these small steps push markets away from the status quo and toward formulas that protect not only your car tires or snack foods, but the water, soil, and people nearby.
2,6-Di-Tert-Butyl-4-Ethylphenol sits on the shelves of many labs and factories across the world. Most folks know it for its role in keeping things from spoiling—manufacturers lean on it as an antioxidant for everything from fuel to rubber. It’s a common sight in industry, but storage isn’t something to take lightly.
During my years working around industrial chemicals, I’ve seen that even stable-seeming chemicals can catch folks off guard if left in the wrong spot. This kind of phenol shares some traits with other organic compounds: it's flammable, and if conditions go sideways, it doesn’t shy away from producing fumes or turning downright nasty to breathe. Safety data point to this risk, and stories from older colleagues still stick in my mind—one guy left a bottle near a window, thinking the dark glass would do all the work. The heat built up, and one cranky Monday morning, the smell in that lab sent everyone scurrying outside. That single mistake cost a day’s work and put the crew at risk.
Some rules are easy to follow, and storing 2,6-Di-Tert-Butyl-4-Ethylphenol is one of them. Keep the drums or bottles tightly closed, away from any sources of heat or open flames. It might sound like common sense, but complacency sneaks in fast. A cool, dry place, shielded from direct sunlight, keeps the compound from reacting. I’ve worked in spaces where folks use an explosion-proof refrigerator; not everyone needs that level, but those with bigger stockpiles benefit from it.
Air exposure has always bugged me more than most. Screw caps and sealable containers cut down on degradation and vapor leaks. In more humid climates or crowded warehouses, I’ve seen silica gel packets tossed into the container’s secondary box—simple, cheap, effective.
Labels disappear, and over time, even smart people start guessing what’s inside. That’s a fast track to trouble. Clear, visible chemical names and hazard symbols take the mystery out of every shelf. Building this habit into routine inventory checks would have prevented several messy spills I’ve witnessed. Training sits right beside good labeling. It may seem repetitive going over the storage protocols each month, but I’ve seen new hires and seasoned workers both get tripped up by skipped steps.
It’s not about fancy technology or expensive gear—just reliable routines. If leaks spring up, fix them the same day. Don’t set bottles right on the floor; use shelving that can resist corrosion in case anything seeps out. Encourage anyone working with these materials to speak up early if a bottle’s gone off-color or starts to swell. This kind of teamwork and vigilance saved us on more than one late shift.
Safe storage of 2,6-Di-Tert-Butyl-4-Ethylphenol boils down to details: keep it cool, dry, closed, and clearly marked. Keep an eye out, trust your nose, and back up each other. These habits protect not just project schedules, but the people behind every batch.
Chemicals like 2,6-Di-Tert-Butyl-4-Ethylphenol stand out in industries that need antioxidants for plastics, fuels, and lubricants. Over the years, producers have shifted their attention to offering larger quantities as industrial users began scaling their projects. These chemicals aren’t just fancy additives—they help keep products from breaking down, so the cost of maintenance and replacement drops. Engineers and formulators look for steady supply chains, not occasional small shipments. Without bulk access, whole production lines can stall. Anyone who’s run up against a hard-to-find intermediate knows that feeling of frustration.
Global suppliers in North America, Europe, and Asia operate facilities that routinely manufacture 2,6-Di-Tert-Butyl-4-Ethylphenol in batches that suit large-scale processing. Chemical traders publish listings every quarter, pointing to multi-ton shipments rolling out of major ports. Real-world orders go through supply chain brokers who vet manufacturers for reliability and environmental compliance. For buyers needing a drum or two, specialty catalogues cover that ground, but it’s the bulk buyers—those seeking several hundred kilograms or more—who drive the largest transactions.
Experience shows that custom synthesis rarely enters the conversation for this particular compound. Established routes exist, prices are stable thanks to predictable raw material costs, and most buyers want certified documentation for each lot. I’ve seen old quotes from five years ago that match recent pricing, showing how mature this supply line has become. If demand spikes—for instance, after a regulation change that requires better antioxidant coverage in plastics—manufacturers can usually scale up within a few weeks.
Compliance rules matter. Producers must register with chemical safety boards, and REACH certification plays a big role with European buyers. If I were hunting for bulk quantities, I’d pay close attention to shipping labels, batch analysis sheets, and SDS files. Fake product finds its way into lesser-known channels. Reputable trade platforms now gatekeep sellers, and multinational manufacturers typically publish lab results to help buyers verify identity and purity.
Handling and storage bring their own sets of headaches. This chemical won’t explode at room temperature, but it needs to stay dry and out of sunlight. Any large-quantity purchaser will have protocols for spill response and waste disposal. News stories over the past decade have spotlighted fires and contamination issues in warehouses that cut corners on storage. Talking to staff who run warehouses, I’ve picked up some best practices—label every drum in plain sight, test drums coming off each truck, and run monthly safety drills.
Shortages sometimes hit after weather events (a hurricane in a key shipping region, for example) or during major regulatory changes. Most buyers have contingency plans that include dual sourcing and strategic stockpiling. Pooling resources with nearby manufacturers offers another path, as does contracting with logistics specialists who know customs paperwork and chemical-specific shipping rules. Regulatory agencies could improve transparency between suppliers and buyers by beefing up online registries. Industry groups already encourage members to share inventory info in real time, cutting down on hoarding and smoothing out hiccups.
Bulk availability ultimately comes down to who’s asking and how far they’re willing to look. Buyers who network at industry tradeshows and vet their sources usually secure steady supplies, even during crunches. Relationships matter as much as product specs or price per ton, especially for a staple like 2,6-Di-Tert-Butyl-4-Ethylphenol.
| Names | |
| Preferred IUPAC name | 2,6-di-tert-butyl-4-ethylphenol |
| Other names |
2,6-Bis(1,1-dimethylethyl)-4-ethylphenol 4-Ethyl-2,6-di-tert-butylphenol Ethanox 330 Ethyl-2,6-di-tert-butyl-p-cresol DBPC Irganox 100 Antioxidant 33 |
| Pronunciation | /ˈtuː sɛks daɪ tɜːrt ˈbjuːtɪl fɔːr ˈɛθɪl fiːnɒl/ |
| Identifiers | |
| CAS Number | 4130-42-1 |
| 3D model (JSmol) | `3DModel__JSmol__string: "C=C(C(C)(C)C1=CC=C(C)C(C)(C)C1)C"` |
| Beilstein Reference | 1711036 |
| ChEBI | CHEBI:132610 |
| ChEMBL | CHEMBL159897 |
| ChemSpider | 8354 |
| DrugBank | DB14074 |
| ECHA InfoCard | ECHA InfoCard: 100.018.636 |
| EC Number | 201-835-0 |
| Gmelin Reference | 77400 |
| KEGG | C07521 |
| MeSH | D000081226 |
| PubChem CID | 79044 |
| RTECS number | SD6475000 |
| UNII | 7T09U058W6 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C16H26O |
| Molar mass | 206.32 g/mol |
| Appearance | White to Off-White Crystalline Powder |
| Odor | Characteristic |
| Density | 0.948 g/cm3 |
| Solubility in water | insoluble |
| log P | 3.88 |
| Vapor pressure | 0.000017 mmHg (25°C) |
| Acidity (pKa) | 11.7 |
| Basicity (pKb) | 10.40 |
| Magnetic susceptibility (χ) | -44.09·10^-6 cm³/mol |
| Refractive index (nD) | 1.521 |
| Viscosity | 7000 mPa.s (25 °C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 379.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -389.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7136.7 kJ/mol |
| Pharmacology | |
| ATC code | A01AB02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS labelling: `Warning; H315; H319; H335; P261; P305+P351+P338` |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H410 |
| Precautionary statements | P210, P261, P273, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1,2,0, |
| Flash point | 110 °C (230 °F; 383 K) |
| Autoignition temperature | 510 °C |
| Lethal dose or concentration | LD50 oral rat 3300 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3300 mg/kg (oral, rat) |
| NIOSH | SF8575000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | 30 mg/kg |
| Related compounds | |
| Related compounds |
2,6-Di-tert-butylphenol 2,4,6-Tri-tert-butylphenol Butylated hydroxytoluene Ionol 2,6-Di-tert-butyl-4-methylphenol |