Chemists first looked to benzoquinone derivatives in the search for stable oxidants. The backbone of these molecules, the quinone ring, started cropping up in various chemical processes and studies over the twentieth century, with some researchers adding bulky tert-butyl groups at key positions. The 2,6-di-tert-butyl-1,4-benzoquinone structure evolved in laboratories that aimed to get around air sensitivity and self-polymerization issues seen with basic quinones. Each tert-butyl group offered a shield that let the quinone hang around longer before breaking down, opening doors for uses that simple benzoquinone never could handle. Over decades, chemists poured over this compound, both for its organic stability and as a platform to build new, bolder materials.
2,6-Di-tert-butyl-1,4-benzoquinone shows up in the chemical supply chain as a bright yellow crystalline solid. Bulk producers usually market it for research or specialty manufacturing, targeting labs that need a mild but dependable oxidizing agent. Big pharma, research universities, and the fine chemicals industry see it as a workhorse for pushing along tricky reactions. Shelf-stable, robust in storage, and easy to weigh, it doesn’t break down or brown as quickly as the parent benzoquinone. Supply catalogs often list it alongside other quinone derivatives, catering to those who want practical benefits instead of chasing down rare or obscure compounds.
A quick glance at this compound shows a molecular structure built for staying solid at room temperature. The heavy tert-butyl arms attached to the core bring up the melting point and improve the material’s thermal stability, making it less eager to decompose under mild heating. Handling becomes straightforward since the solid doesn’t sublimate away. The molecular weight clocks in higher than plain benzoquinone, and its low volatility means less worry about vapor hazards in the lab. Chemical reactivity leans on the classic ability of quinones to shuttle electrons, yet the tert-butyl groups add steric hindrance that blocks unwanted side reactions. This blend of features lets users focus on the chemistry without fighting constant decomposition or excessive odor.
Quality control steps highlight purity, usually reported over 98% for research applications. The color should appear distinctly yellow, not brown or dull orange, since that often signals degradation. Chemical suppliers print batch details, storage recommendations (keep sealed, dry, avoid strong acids or bases), and hazard warnings. Most bottles or pouches feature GHS labeling, pointing out that the material irritates skin and eyes on contact. Reputable suppliers include a Certificate of Analysis to back up purity claims, listing melting point, GC-MS, NMR, and HPLC traces. This transparency makes it easier for labs to trust every batch without a detective hunt for contaminants.
Lab-scale synthesis relies on oxidation of 2,6-di-tert-butyl-1,4-hydroquinone. Modern chemists prefer using oxidants like silver oxide, ferric chloride, or even simple air under basic or neutral conditions. Pursuing greener chemistry, some groups look for less toxic oxidants and solvent systems, trying to keep waste streams cleaner and easier to neutralize. Once the reaction ends, the product gets cracked out using solvents like ether or dichloromethane, followed by crystallization. Careful handling during this phase avoids overoxidation or reduction by light, which can mess with the yield and purity. This hands-on, iterative process keeps air exposure and moisture down, since even small lapses can dent product quality.
The molecule does its best work in redox chemistry. Its strong but manageable oxidizing power pushes organic and inorganic transformations where milder agents fizzle out. Compared to plain benzoquinone, the tert-butyl version fights off unwanted polymerization and tars, giving cleaner reaction profiles. Chemists often tweak the molecule, trading tert-butyl groups for other bulky moieties, or attaching halogens, chasing the right mix of reactivity and selectivity for new syntheses. The core 1,4-benzoquinone ring supports a range of nucleophilic additions and cross-coupling strategies, carrying the tert-butyl shields throughout. It stands as the go-to choice for precise, low-waste oxidations in free radical chemistry and organic synthesis.
This compound picks up a range of names depending on who’s talking. Some catalogs stick to the full IUPAC mouthful: 2,6-di-tert-butyl-1,4-benzoquinone. Others shorten it to DTBQ or simply call it alkylated benzoquinone. In patent literature, names like DBBQ or its systematic identifiers show up. Picking through old research, you might see “Duroquinone” or trade names dreamed up by specialty chemical formulators. Chemistry students remember that every group adds a new nickname, so researchers keep synonyms close to hand, hunting across different journals and supplier lists.
Handling 2,6-di-tert-butyl-1,4-benzoquinone means focusing squarely on safety from the start. As a quinone, it can provoke skin and respiratory irritation, so gloves, eye protection, and lab coats stop accidental exposures cold. Well-ventilated benches and fume hoods cut down inhalation risk, especially for bigger batches or scale-up projects. Spills get swept up with inert absorbents, not water, since moist conditions spark slow degradation. Waste disposal lines up with hazardous organics protocols, keeping quinones out of the regular trash stream. Detailed risk assessments in chemical safety data sheets help newcomers and veterans keep these standards front and center every time a bottle gets opened.
The most common use ties back to organic synthesis, where clean, reliable oxidants matter more than ever. DTBQ drives antimalarial agent creation, flavors synthesis, antioxidant chemistry, and radical chain reactions. In teaching labs, the robust color shift makes it great for redox titrations and starter experiments, showing students how color tracks electron movement in real time. Industrial teams run DTBQ through resin and polymer chemistry cycles, looking for out-of-the-box crosslinkers that can handle heat and UV. Electrochemical research circles keep it on hand for battery cathode prototypes and model studies of quinone-based redox flow cells. This chemical’s reach stretches past textbooks, making a mark in fields as far-flung as materials science and drug lead discovery.
Research teams push hard to uncover new reactions that benefit from DTBQ’s unique blend of oxidation power and stability. Universities chase milder conditions for C–H activation, replacement of harsher oxidants, or even as a mediator in transition metal catalysis for bond formation. One trend looks at merging DTBQ into flow chemistry, bringing safer, scalable oxidation steps to the pilot plant. Teams track how every tweak to the quinone skeleton changes selectivity, hoping to capture the next big jump in green synthesis. These design efforts funnel through international conferences and open-access journals, drawing graduate students and postdocs into the hunt for more productive reaction partners or unexpected biological activities.
Toxicologists take these quinone derivatives seriously. With certain quinones, repeated exposure means more than skin rash—it sparks cell stress in sensitive tissues. DTBQ shows moderate toxicity in cell cultures, with research pointing to oxidative stress effects on liver and kidney cell lines. Acute exposure studies in rodents outline dose-dependent impacts that steer safety margins in the workplace. Regulatory agencies keep a close watch for data on chronic effects or potential long-term damage, weighing every claim against peer-reviewed evidence. By understanding and sharing these risks, chemists and employers cut preventable exposures, making research both productive and safer for everyone involved. That way, innovation doesn’t have to run ahead of occupational health.
Looking ahead, 2,6-di-tert-butyl-1,4-benzoquinone could evolve from lab staple to key ingredient in cleaner, more selective oxidation processes. Researchers see it as a candidate for greener chemistry, where it stands as a replacement for more toxic or unstable oxidants. Its resilience offers a platform for new materials in redox-active polymers, battery prototypes, and even as a model for biological electron transfer studies. The push for safer, recyclable, and high-yield chemical solutions keeps drawing new attention from scientists needing performance without the hazards of older quinone stocks. With every new publication, the compound’s reach grows, pulled forward by the twin engines of safety and efficiency in demanding scientific fields.
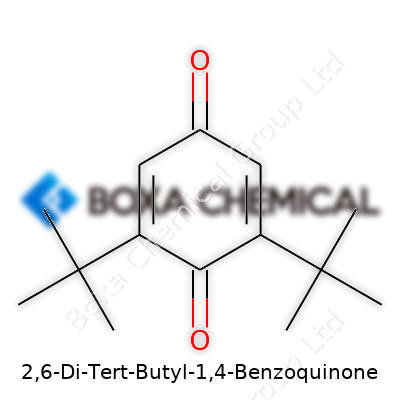
Chemical names often make people’s eyes glaze over, yet every part tells a story. Take 2,6-Di-Tert-Butyl-1,4-Benzoquinone, for example. At its core, this molecule builds on benzoquinone—a simple ring with a reputation for easy redox chemistry. Attaching tert-butyl groups at positions 2 and 6 bulks up the skeleton, changing both its reactivity and its physical character.
Picture a six-carbon ring—this represents the backbone, known in chemistry as a benzene ring. At positions 1 and 4, swap out hydrogens for oxygen atoms double-bonded to the carbons. That shift from a classic benzene to a quinone changes the molecule’s electron dance, opening the door for unique interactions. Now, at carbons 2 and 6, attach two big, branched tert-butyl groups—imagine a central carbon joined to three methyl groups. These tert-butyl groups stick out like elbows at a formal dinner, taking up a lot of space.
With big side chains flanking the structure, this molecule no longer has that flat plan of a simple benzoquinone. Those groups force a different packing pattern in solid form and keep the molecule from easily stacking or undergoing certain side reactions. I’ve worked in labs where this makes it easier to isolate products, or sometimes creates stubborn hurdles during purification.
Plenty of chemists rely on small changes to a molecule to solve big problems. Bulky tert-butyl groups stabilize the benzoquinone core in air and solution, since chemical crowding guards against attack from eager nucleophiles or stray radicals. This often comes up in antioxidant chemistry—those big arms absorb the wear and tear meant for more vulnerable parts of the molecule.
Scientists figured out how to use this compound to track electron movement, to probe enzyme mechanisms, or tune electronic properties in organic devices. Early work on non-aqueous batteries saw researchers turn to 2,6-Di-Tert-Butyl-1,4-Benzoquinone for its stability. In my own experience, choosing a quinone without bulky groups can lead to fast breakdown and unreliable measurements—a headache for anyone pushing towards real-world solutions.
The chemical structure has strengths and limits. Those big tert-butyl arms offer more than just stability; they also change solubility. In solvents like benzene or hexane, you get better mixing, but water rejects the molecule almost entirely. That brings hurdles for green chemistry or environmental applications, where water-friendly molecules take priority.
If the world wants cleaner and safer quinones, it starts with smart design. Chemists today try to swap out some of that bulk for just enough protection, maybe using short chains or sugar-like wings, aiming for balanced life between air stability and water solubility. This often means striking a compromise: enough bulk to stop unwanted chemistry, but not so much that the molecule stays too stubbornly in oil-based worlds.
Real progress builds from knowing the gritty details. The full IUPAC name of 2,6-Di-Tert-Butyl-1,4-Benzoquinone is 2,6-Di-tert-butylcyclohexa-2,5-diene-1,4-dione. Its formula is C18H26O2. Its structure can be visualized as a para-benzoquinone core, flanked symmetrically by two tert-butyl groups, and two double-bonded oxygens on the ring—each detail creating unique physical and chemical fingerprints.
Tackling the needs of advanced batteries, green catalysts, or medical applications all start with tiny changes to molecules like this. Structure reveals not just what a molecule looks like, but points toward solutions, both in the lab and beyond. Every chemist I know has tried, failed, and adjusted these designs, hunting for the right balance. That work continues, driven by equal parts frustration and hope.
Looking at the chemical world, 2,6-Di-Tert-Butyl-1,4-Benzoquinone usually shows up as a yellow-orange powder, catching the eye of anyone who spends time around labs or industrial settings. The molecule, armed with bulky tert-butyl groups, stands out for its stability and unique redox properties. Many chemists, whether in academia or large-scale manufacturing, reach for this compound when they need both strength and reliability in reactions that involve electron transfer.
No organic synthesis textbook or research bench can escape the impact of quinones. 2,6-Di-Tert-Butyl-1,4-Benzoquinone shines as an oxidizing agent. Trying to convert alcohols to aldehydes or ketones? This compound gets the job done without letting side reactions run wild. Its stability in both open air and solution gives laboratories and production plants the confidence to scale up reactions. For example, many pharmaceutical projects draw on this quinone during their quest to build complex molecules cleanly and efficiently.
Chemists who spend afternoons controlling stubborn radical reactions often reach for well-chosen antioxidants or radical scavengers. Here, 2,6-Di-Tert-Butyl-1,4-Benzoquinone steps in, mopping up excess radicals and improving yields in polymer chemistry and material science. Polymers, ranging from protective coatings to medical devices, benefit from a little help as they form long chains. Adding a controlled amount of this compound keeps unwanted side reactions under control, protecting both structure and function.
Scientists don’t just look at molecules – they want to understand how they change. 2,6-Di-Tert-Butyl-1,4-Benzoquinone often serves as a reference compound in electrochemistry. Measurements of electron transfer rates, redox potentials, or simply the ability of a system to move electrons all draw on its predictable behavior. This supports quality work in battery research, sensors, and environmental analysis, helping specialists develop diagnostics, monitor pollution, or push the frontier on green energy devices.
Many finished goods, whether lubricants, plastics, or fuels, face threats from air and light. Oxidative degradation breaks down bonds and destroys performance. Here, the antioxidant properties of 2,6-Di-Tert-Butyl-1,4-Benzoquinone matter to producers. Adding measured doses preserves shelf life and functionality. Those who grew up in industrial labs know the damage that water, oxygen, or sunlight can inflict ; a smart choice of stabilizer like this quinone can make the difference between a short-lived product and one that survives the test of time.
Getting results in the lab or on the production line doesn’t mean cutting corners. Like many organic chemicals, 2,6-Di-Tert-Butyl-1,4-Benzoquinone poses challenges if handled carelessly. Reading safety data sheets, using gloves and goggles, and keeping good ventilation minimizes exposure. Larger manufacturers benefit from investing in waste management and recovery methods that curb emissions. Everyone working with this compound, from undergraduate students to senior engineers, shares responsibility for safe and sustainable use.
Stepping into the future, efficient, reliable, and environmentally conscious applications of specialty chemicals like 2,6-Di-Tert-Butyl-1,4-Benzoquinone drive progress. Better training, closer industry-academia partnerships, and open communication can foster an environment where new applications and safer practices develop side by side, keeping both people and profits in balance.
2,6-Di-Tert-Butyl-1,4-Benzoquinone usually shows up as yellowish crystals, not exactly what most people expect when they hear the word "quinone." With big, clunky tert-butyl groups sticking out on the ring, it stands up pretty well to the air compared to lighter, simpler quinones. Still, it reacts with oxygen, moisture, and light over enough time. At a university lab, I saw a jar left out on a shelf pick up a brown tint and clump together over a few weeks, turning it pretty much useless for any precise research. Waste of money and time, all because someone thought "it looks stable enough."
I learned early to pay more attention to the way bottles lined the back shelves than to the fancy instruments out front. A compound like this doesn’t need white-gloved treatment, but it punishes laziness. I always found it sat best in amber glass, with a solid screw-top: easy way to cut down the daily blast of sunlight from lab windows. Plastic never gave the same protection, and any thin plastic jar would sweat fumes through the lid, leaving a stain you never get off your fingers. Thick glass wins.
For moisture, nobody enjoys opening a jar to find the contents caked. Silica gel packs thrown in with the bottle made a difference every single time. Someone once tried rice, but rice holds onto water more than silica, and it just made things smell weird after a few weeks. So, silica gel it is. Weird tip: always swap out the gel if it starts to turn pink—they’re cheap and make all the difference.
This benzoquinone runs fine at room temperature by most guides, but I’ve known research teams who pop it in a cool, dark cabinet below twenty degrees, if possible. No need for a freezer—the frost and dripping condensation can make life worse. Just a cabinet out of direct sun and away from the radiator. Any time the temperature goes up and down, crystals will start to clump, and after enough cycles, you get degradation.
Leaving this quinone near reducing agents, bases, or acids invites trouble. In one lab, a careless mix-up with a nearby open bottle of sodium borohydride triggered a fizzing mess that cost days of clean-up—not recommended. Neat rows, separated by hazard class, keep accidents to a minimum. If you work in a university lab, pestering people to keep things organized isn’t just being uptight; it saves money, time, and headaches. Regulation is only as good as daily habits.
Labels fade, handwriting smudges, and nobody remembers "which batch came when" after a month. Scanning QR codes on bottles helped one of our teams stay on top of stock rotations—nobody wants to discover expired material after it's in the reaction mix. Expiry dates and the date opened reduce guesswork. A digital log, even just a spreadsheet, lets everyone notice trends. If purity dips or the color goes off faster than last year’s batch, that means it's time to revisit storage habits.
Smart storage isn't just for the safety inspector; it means saving resources and cutting down on lab drama. Every wasted jar becomes a story, not just a statistic. Care pays off, every time.
Anyone who’s worked with 2,6-Di-Tert-Butyl-1,4-Benzoquinone in a lab knows its potential isn’t limited to just research discoveries. It’s a strong oxidizer and brings some serious health risks along. I remember my first job out of college, prepping benches at a synthetic chemistry lab. The supervisor pointed at a yellow powder, warning, “Watch out. That stuff can burn holes in your gloves and your nose.” She wasn’t exaggerating. This benzoquinone can cause skin and eye irritation, and breathing in dust triggers coughing and burning in your airways.
It’s not only about short-term effects, either. Some studies link long-term exposure to oxidative stress in cells, which might lead to permanent tissue damage. Working in chemistry, I’ve had close calls with harsh reagents, and accidents with substances like this make you rethink getting too casual. The compound’s volatility makes vigilant handling an ongoing necessity.
Clean nitrile or neoprene gloves, a properly fitted lab coat, safety goggles, and a face shield form the backbone of protection. Cotton gloves melt or tear too quickly and offer little defense. I learned to double-glove after a spilled bottle soaked through a single pair. Dry powder can sneak around your fingers, so always check for gaps or rips in gloves.
Don’t skip on eye protection. I’ve seen what happens when a neighbor pops a flask and ends up at the emergency room; a splash in the eye moves fast. Tinted goggles also help since some quinones break down under light, but nothing beats a full face shield for mixing or weighing powders.
Breathing in even small amounts of this powder feels rough, like you swallowed sandpaper. Fume hoods keep dust and fumes from hitting your lungs. Always run the sash low, as close to the work as you can manage, and make sure the airflow gauge is in the green. If you ever smell or taste anything unusual, leave and check for leaks.
In my experience, keeping stock bottles tightly capped and stored away from light and heat stops accidental exposure and preserves stability. I always label secondary containers clearly. Once, I saw a bottle on a communal shelf, no label, and it took hours sorting out what it was and whether it needed disposal. One slip and you could spark a reaction with incompatible substances like acids or strong bases.
Pouring waste quinone down the sink spreads pollution and can ignite plumbing—literally happened at an old teaching building, shutting down eleven labs for weeks. Dedicated containers marked for hazardous waste, pick-up by trained staff, and updating Safety Data Sheets on hand all help everyone keep track of what’s around.
Spill kits work best when they’re fresh and nearby. I prefer kits that feature absorbent powders over paper towels, since these contain and neutralize spills quickly. I train new techs to know where these kits are, not just for this compound but for every hazard in the room. If somebody coughs or shows rashes, get them out, flush with water, and call emergency services fast—speed is always your friend.
Most accidents I’ve witnessed don’t happen because people don’t know the rules. They happen when someone gets comfortable or careless. Refresher training and visible reminders—printed signs, checklists at every bench—remind everyone that safety steps aren’t just about rules, but real lives. Taking careful steps limits risk and forms habits that stick, long after the benzoquinone is gone.
Anyone who's ever picked up a bottle of chemicals for research or manufacturing knows purity changes everything. Contaminants can derail entire experiments, throw off yields in reactions, or even trigger safety incidents. For 2,6-Di-Tert-Butyl-1,4-Benzoquinone, which folks often call DTBQ for short, purity marks the difference between a trusted reagent and a risky gamble. Most suppliers offer this compound at no less than 98% purity. Higher grades—upward of 99% or even HPLC-pure varieties—do exist for labs with critical applications. Going below 98% stirs trouble in fields like organic synthesis, as extra by-products confuse results or introduce unknown hazards.
In organic chemistry and materials science, small impurities bring outsized problems. I've worked with quinones in redox processes, and sometimes a single unknown peak in the spectra hints at an impurity causing side reactions. Experiments seem straightforward, but yields slip, or products darken unexpectedly. Getting a high-purity DTBQ keeps reactions predictable. We count on benchmarks for melting points and reactivity; any hitch due to impurity means days wasted troubleshooting. These hiccups scale up in manufacturing too. Imagine a pharma batch failing quality control because of a trace contaminant. Every point of purity lost can multiply losses in those settings.
Industry and academia rarely work at the same scale. In my experience, research labs pick up DTBQ in small bottles, usually 1 gram or 5 grams. These sizes fit routine syntheses or analytical work. Once projects scale up—think pilot plants or bulk manufacturing—requests shift to larger bottles, bags, or drums. Vendors frequently stock sizes like 25 grams, 100 grams, and even kilogram-scale pails. Bulk orders can run to 5 or 10 kilograms, shipped in lined containers to keep the compound stable during transport. I've seen orders packed tightly to avoid moisture and light, both of which can spoil quinones.
In most labs, the same container of DTBQ passes from hand to hand, moving from gloveboxes to bench tops. This material shows some sensitivity to air and light, especially at the edges of its shelf life. Resealable amber glass bottles offer the best protection. For those handling bigger batches, sealed metallic cans add peace of mind. It’s surprising how fast a gram bottle of quinone can darken if left under bright lights, so it pays to portion out only as much as needed and return the rest to storage promptly. Moisture eats away at both purity and performance, so desiccators never sit empty when this compound’s around.
Reliable quality starts with trusted sources. Not every supplier treats consistency as a top priority, but repeatable purity and accurate packaging build trust fast. Certificates of analysis help vet batches, laying out trace metal content and confirming assay values. These reports have helped me filter out subpar lots many times. If a technical problem pops up in the lab, purity and packaging details often tell the story. Tech support from suppliers who understand both the chemistry and practical needs of their customers goes a long way.
Investing in batch-to-batch quality checks stops trouble before it starts. Suppliers who update customers about any process changes earn loyalty in the long run. Today’s push for greener, safer compounds only raises the standard for purity and handling. More labs opt for smaller, fresher batches. Bulk buyers press for plain-spoken batch data and secure packaging. For anyone using DTBQ, the conversation around purity and packaging stays ongoing—a real partnership between chemist and supplier that demands honesty, experience, and continual improvement.
| Names | |
| Preferred IUPAC name | 2,6-di-tert-butylcyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˌtuː sɪks daɪ tɜːrt ˈbʌtɪl wʌn fɔːr ˌbɛnzoʊkwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | “719-22-2” |
| 3D model (JSmol) | `3Dmol.js?chem:JSmol&id=C12H18O2` |
| Beilstein Reference | 92434 |
| ChEBI | CHEBI:52714 |
| ChEMBL | CHEMBL107781 |
| ChemSpider | 21106373 |
| DrugBank | DB08636 |
| ECHA InfoCard | 20-211-230-3 |
| EC Number | 204-327-1 |
| Gmelin Reference | 81262 |
| KEGG | C16582 |
| MeSH | D017967 |
| PubChem CID | 69703 |
| RTECS number | DC2625000 |
| UNII | X3KQ4ENJ7M |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C14H20O2 |
| Molar mass | 222.32 g/mol |
| Appearance | Yellow to greenish-yellow crystalline powder |
| Odor | Pungent |
| Density | 1.07 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 2.86 |
| Vapor pressure | 2.5 mmHg (25 °C) |
| Acidity (pKa) | 15.8 |
| Magnetic susceptibility (χ) | −19 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.568 |
| Viscosity | 15 cP (20°C) |
| Dipole moment | 2.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 327.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -322.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1429.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261-P280-P305+P351+P338-P304+P340-P405-P501 |
| NFPA 704 (fire diamond) | 1-1-0-** |
| Flash point | 113 °C |
| Autoignition temperature | 455 °C |
| Lethal dose or concentration | LD50 oral rat 890 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 800 mg/kg |
| NIOSH | DH6650000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20-50 mg/L |
| IDLH (Immediate danger) | Not listed |