Chemists first began exploring benzoquinone derivatives in earnest during the early twentieth century, drawn by their roles in natural redox processes. 2,5-Dimethoxy-1,4-Benzoquinone traces its origins to early plant physiology studies, where this compound turned up as a pigment in several botanical extracts. Researchers identified and separated it during attempts to pinpoint electron transport mediators, marking an era where knowing the structure and reactivity of these small, para-quinone molecules meant unlocking clues about plant cellular respiration. The compound became a scientific fixture, not due to overwhelming commercial appeal, but thanks to its intricate participation in redox chemistry. Many fundamental methods in organic synthesis, including oxidative coupling and enzymatic activity tracing, leaned on the properties revealed by studies of quinones much like this one.
2,5-Dimethoxy-1,4-Benzoquinone crops up in both research labs and select specialty manufacturing processes. The molecule carries two methoxy groups attached to the aromatic ring, helping tweak its electron-withdrawing ability. This subtle molecular tuning gives it qualities that other benzoquinones lack, turning the compound into a useful model for developing new oxidation catalysts, dyes, and mediators for enzymatic reactions. In my own experience, its high demand among electrochemists comes from that balance between stability and reactivity. Whether dissolved in solvents or supplied in crystalline form, each batch comes with a distinct reddish-brown hue that signals its oxidized nature, ready for redox cycling or further chemical modification.
2,5-Dimethoxy-1,4-Benzoquinone appears as a red to orange crystalline solid, standing out from simpler para-benzoquinones thanks to those methoxy substitutions. Its melting point hovers around 144°C, and it dissolves moderately well in polar organic solvents such as ethanol, methanol, and chloroform. Water solubility stays low, a reflection of the molecule’s hydrophobic aromatic ring. Chemically, the two methoxy groups shore up the quinone core, providing electron-donating effects that make this compound a bit more resistant to reduction. Even still, it eagerly participates in single-electron transfer and reduction–oxidation cycles, properties that researchers depend on during anaerobic oxidase enzyme studies and bioelectrochemical experiments. The solid stores best in airtight containers at cooler temperatures to avoid moisture or light-triggered degradation.
Manufacturers typically provide 2,5-Dimethoxy-1,4-Benzoquinone at purity grades greater than 98%, sufficient for most analytical and preparative tasks. The substance lands in labeled amber-glass containers to block light, with labels indicating lot number, net weight, purity, and manufacturer address. Hazard and precautionary statements highlight the compound’s oxidizing nature. Labels conform to Globally Harmonized System (GHS) requirements, showing proper pictograms and signal words to back up local safety regulations. Most suppliers back each shipment with a Certificate of Analysis and Safety Data Sheet, essential for ensuring traceable handling from lab bench to industrial floor.
Manufacturers rely on precise oxidative methylation to synthesize this compound, feeding 1,4-dimethoxybenzene through a controlled oxidation—a process often using reagents like silver oxide or ceric ammonium nitrate. The reaction selectively draws off two hydrogens, installing the characteristic diketone structure at the heart of the quinone. Product isolation involves solvent extraction, evaporation, and repeated crystallization to reach the right purity grade. Each step calls for careful attention to temperature and exclusion of air or water, since quinones tend to degrade or form byproducts under uncontrolled conditions. Scale-up from gram to kilogram quantities stays challenging because yield and purity can dip if reaction variables drift off-spec.
Chemists often exploit 2,5-Dimethoxy-1,4-Benzoquinone’s reactivity for broader synthetic goals. The molecule’s carbonyl groups readily participate in nucleophilic addition and reduction, offering routes to functionalized catechols or hydroquinones. Under mild reducing conditions, the quinone core converts to the corresponding hydroquinone, which finds use as an antioxidant or a precursor to specialty dyes. The methoxy groups open the door for substitution reactions, yielding derivatives that researchers screen for new catalytic properties or as model compounds in organic electronics. The compound’s ease of modification pushes it into research on artificial photosynthesis, where electron transfer chains demand stable, reversible redox centers.
Publications and commercial listings reference 2,5-Dimethoxy-1,4-Benzoquinone by several names—“2,5-DMBQ,” “2,5-Dimethoxy-p-benzoquinone,” and sometimes just “DMBQ.” I’ve seen variations depending on language and supplier; some catalogues prefer IUPAC nomenclature, listing it as “2,5-dimethoxy-1,4-benzoquinone,” while others stick to briefer trade abbreviations. Awareness of synonyms smooths the process of combing through patent records or cross-checking toxicity databases, as missing a variant could mean missing crucial hazard or regulatory information.
Handling this compound means recognizing its oxidant potential. Prolonged skin contact leads to irritation, and inhalation of dust or vapors may trigger respiratory discomfort. In my years running bench-scale reactions, a fume hood and gloves became second nature every time the container opened. Labs observe routine disposal of spills via non-reactive absorbents, keeping all waste in labeled, contained solvent drums for specialized collection. Avoiding open flames and overheating prevents decomposition and toxic byproduct formation. Storage guidelines recommend dark, dry, low-temperature spaces, cutting down the odds of unwanted reactions during warehousing. Training on the substance covers emergency first aid for exposure, highlighting eye-wash protocols and the importance of PPE. Up-to-date understanding of local, national, and international transport codes keeps things compliant during shipping.
Researchers and industry professionals put 2,5-Dimethoxy-1,4-Benzoquinone to work as a redox mediator, especially in bioelectrochemistry and enzymatic system studies. Its unique electron flow behavior makes it a tool for probing electron transfer mechanisms in plant biochemistry and microbial metabolic pathways. Academics have leveraged it for studying oxidative stress or as a structural analogue in new drug development screens. The dye and pigment industry values its vivid color and reversible redox cycling, using it as an intermediate during specialty pigment synthesis. Undergraduate teaching labs sometimes turn to this compound when illustrating redox equilibria, integrating its properties into practical, hands-on demonstrations for chemistry students.
Ongoing R&D trends explore 2,5-Dimethoxy-1,4-Benzoquinone for its value in developing next-generation organic electronic materials and as a benchmark molecule in artificial energy conversion. Redox flow batteries demand stable, easily modified electron carriers, and this molecule fits that search. Some projects have examined tailored derivatives for improved stability and charge-transfer in energy storage systems, turning from theoretical modeling to bench-scale cell tests. On the biological front, studies map out its impact as both a substrate and effector in enzyme systems—work that feeds into agricultural biotechnology and the screening of new antifungal or antibacterial agents. Pharmaceutical chemists also adapt this chemical core, investigating potential anti-cancer and anti-inflammatory activity, taking cues from reports that draw connections between quinone structure and cellular stress responses.
Toxicologists catalog effects on both acute and chronic exposure to 2,5-Dimethoxy-1,4-Benzoquinone. Data from in vitro studies reveal that this quinone can prompt oxidative damage at high concentrations, interfering with cellular electron transport chains. Animal studies demonstrate localized irritation and, in excessive scenarios, organ-specific toxicity related to redox disruption, though these findings depend on route and duration of exposure. Regulatory agencies include this compound on lists requiring strict workplace exposure monitoring and call out proper disposal to shield both public and environmental health. Updates to safety data often echo broader findings from related chemicals, urging lower occupational exposure limits as more is known. Continued vigilance from industrial hygienists and researchers matters, especially since chemical analogues move through labs and production facilities in rising volumes.
As the fields of renewable energy and green chemistry evolve, compounds like 2,5-Dimethoxy-1,4-Benzoquinone hold promise as key players. The molecule’s reliable redox chemistry gives researchers a launch pad for developing safer, longer-lasting organic battery components. Modifications aimed at boosting solubility, or shifting redox potential, already point toward new classes of flow battery electrolytes—technology that underpins scalable energy storage. In laboratory medicine, the molecule stands as a scaffold for next-generation diagnostic and therapeutic agents, especially as scientists zero in on the redox regulation in disease. Rules surrounding manufacture, transport, and disposal will keep shifting as more sees daylight on health and environmental influence. As research budgets grow for alternative energy, synthetic biology, and biomedical innovation, the relevance of 2,5-Dimethoxy-1,4-Benzoquinone expands, promising a role that goes well beyond its traditional boundaries in basic organic chemistry.
2,5-Dimethoxy-1,4-benzoquinone sounds like the sort of thing you’d only hear about in a chemistry lecture, but this compound quietly plays a role in several areas of research and technology. I remember reading about compounds like this back in university, feeling lost in their chemical names, until I started seeing how they help drive bigger stories in science and medicine.
Researchers use this chemical mostly for its ability to shift electrons. In plain talk, it’s an electron acceptor. This quality makes it valuable in studying energy transfer processes, especially when working with photosynthetic systems. Think of it as a tool for scientists to mimic and break down how plants turn sunlight into energy — a process that fuels nearly every ecosystem.
I once spoke to a graduate student who explained how this compound helped map out electron flows across membranes in bacteria. Without it, the lab struggled to simulate natural photosynthesis in a meaningful way. With this molecule, their experiments suddenly made sense.
Beyond basic science, this chemical shows up in early drug discovery and toxicology screens. Its predictable reactions supply a stable benchmark for measuring oxidative stress or spotting antioxidant activity in other compounds. Anyone who has followed news on antioxidant supplements knows those claims often hinge on how well a molecule holds up against oxidative damage. Lab work often falls back on 2,5-dimethoxy-1,4-benzoquinone as a control when testing new antioxidants.
Some read that and ask, "So, could this actually become a drug?" Right now, there’s no sign of it on pharmacy shelves. The real value lies in giving a baseline to judge whether new molecules do anything special. Chemists lean on it, a bit like a carpenter relies on a level to see if a frame stands straight. Even in routine cancer cell testing or screening for heart medication, this compound may be part of the toolkit.
Away from labs, benzoquinones can help craft molecules for organic electronic devices. Some manufacturing teams experiment with 2,5-dimethoxy-1,4-benzoquinone as a piece in larger systems, such as organic solar cells. Since it shuttles electrons well, engineers look for ways to plug it into circuits designed to harvest light or move charge efficiently. Nothing has made it to mass production yet, but the push for greener tech keeps this sort of research moving forward.
Every chemical comes with upsides and downsides. Handling benzoquinone derivatives demands respect. Strict lab protocols exist for storage and handling because exposure can cause skin and eye irritation. I’ve seen labs where gloves and proper ventilation aren’t just suggested — they’re enforced. Ignoring safety, even for compounds used in low doses or test tubes, leads to accidents.
Using chemicals safely and responsibly runs deeper than keeping researchers healthy. It builds trust in research findings. Laboratories that document safe handling and disposal of substances like this help keep doubts from clouding published studies. This honesty protects everyone, from the undergraduate assistant to the public reading headlines about the next scientific breakthrough.
The uses for 2,5-dimethoxy-1,4-benzoquinone could expand as green energy and biomedical research progress. Supporting ethical access to this compound — along with transparency around safety data — helps level the playing field for young scientists worldwide. Turning ideas into treatments, cleaner fuels, or better electronics often depends on tools like this, which quietly power science out of the limelight.
2,5-Dimethoxy-1,4-benzoquinone doesn’t turn many heads outside the lab, but the formula—C8H8O4—has real weight for scientists who tinker with antioxidants, plant metabolites, and even some antibiotics. Picture a benzoquinone core, a basic six-carbon ring with two double-bonded oxygens, and swap in two methoxy (–OCH3) groups at the 2nd and 5th carbons. This placement isn’t random. Each group brings subtle changes to electron flow, creating effects chemists and biologists keep tapping into, from electron-transfer reactions to enzyme inhibition. That arrangement fuels plenty of research, even shaping work on drugs and fungal resistance.
People joke about obscure chemistry facts, but knowing the formula C8H8O4 is more than trivia. In any lab setting, you need the right numbers to avoid dangerous mistakes. A wrong calculation with reagents or experimental plans can have serious consequences, including wasted funding, lost experiments, or even safety incidents. I’ve sat at the bench, pen in hand, flipping through references and sometimes catching mismatches between a supplier’s catalog and published literature. Trust in chemical databases grows from repeating checks, cross-referencing, and not cutting corners.
2,5-Dimethoxy-1,4-benzoquinone draws curiosity because of its presence in some natural processes. Scientists have spotted compounds like this in fungi and plants, hinting at hidden biological roles. Botanical chemists have connected benzoquinone derivatives to plant defense signals. Clinical chemists sometimes chase them as models for new therapeutic agents, banking on their redox properties. Understanding the base formula helps experts predict behavior, run assays, and consider modifications. Each atom counts.
Anyone can Google a chemical formula, but open, peer-reviewed sources set the standard. Researchers still cross-check facts in textbooks, databases like PubChem or the NIH’s websites, and with peers. Scientific publishing places strict demands on data integrity, and that culture saves headaches. A few years back, I watched a graduate student spend days tracking a minor error in a supplier’s product description. He painstakingly checked NMR, MS, and IR spectra, and discovered a methyl group out of place. That attention to detail goes unnoticed outside the lab, but it shapes discoveries downstream. One digit in a formula, like moving from C8H8O4 to C8H10O4, means a completely different substance enters the mix.
Basic chemical literacy makes practical sense for more than chemists. The right formula, structure, and nomenclature echo through fields from nutrition science (tracking antioxidants) to green tech (designing electron mediators for sensors or solar cells). Mistakes ripple. Training not just chemists but students, educators, and even journalists to double-check numbers can help prevent information drift. Public science resources with editorial oversight give everyone a fighting chance against misinformation or simple error, reducing chances for accidents or research dead-ends. When accurate chemistry facts circulate, innovation follows without as many costly bumps in the road.
2,5-Dimethoxy-1,4-benzoquinone has a complex name and a place in several scientific discussions. As someone who’s spent significant time working in university labs and collaborating with industrial chemists, I’ve seen this compound get used in organic synthesis and research projects. The question of its toxicity isn’t just academic—many students and professionals handle benzoquinones without a full grip on the risk involved.
The structure of this chemical gives some clues. Benzoquinones tend to act as strong oxidizing agents, and their reactivity translates into biological settings. The body struggles to break down these molecules safely. Most quinones irritate the skin and eyes; some derivatives exhibit cytotoxicity, meaning they can hurt or kill cells. Researchers have flagged several benzoquinone compounds based on their impact on DNA and proteins. In my time working with protective gear in the lab, nobody took handling them lightly. Any spill meant goggles and gloves, quick cleanup, and a lot of respect for what vapors could do to lungs and skin.
Direct tests on 2,5-dimethoxy-1,4-benzoquinone’s toxicity are not as thorough or conclusive as what’s available for more common chemicals, but its parent compound, p-benzoquinone, has a reputation for being a problem. Inhaling benzoquinone vapors leads to symptoms ranging from respiratory irritation to headaches and nausea. It damages tissues on contact, and frequent exposure puts people at risk for dermatitis and even long-term systemic effects.
The methoxy groups on 2,5-dimethoxy-1,4-benzoquinone tweak how the molecule behaves, but they don’t automatically make it benign. Some academic papers suggest it may form reactive oxygen species, which push cells towards oxidative stress. That kind of stress can influence the development of cancer and other diseases.
Experience has taught me that most laboratory accidents involve small lapses—a drop on a glove, or a failed fume hood. For this compound, eye protection, gloves, and a well-ventilated hood aren’t negotiable. Anyone working with it must respect its ability to harm, even in small quantities. The safety data sheets from chemical suppliers don’t mince words about the risks: avoid inhalation, prevent skin contact, and don’t let it near your eyes.
In professional settings, training matters. Too often, new researchers trust that unfamiliar substances have been vetted. That’s a dangerous mindset. No experiment justifies shortcutting safety. Disposal also demands care. Benzoquinones can’t go into regular waste—they find their way into the environment and may disrupt aquatic life. Proper chemical disposal routes protect not just the immediate lab but the wider community.
Regulation and research lag behind reality on many niche compounds. More studies need funding from grants and open-access journals, so better toxicity profiles become available. Industry and academia both benefit from open dialogue, sharing new findings and near-miss incidents. Regular safety training and well-maintained records offer another layer of protection. Peer review works in research—and in lab safety, it catches bad habits before they become disasters.
Every chemical brings opportunity and risk. Recognizing gaps in knowledge and acting with caution keeps people safe. 2,5-Dimethoxy-1,4-benzoquinone isn’t the most dangerous compound in the cabinet, but treating it with real respect is the way to avoid regret.
2,5-Dimethoxy-1,4-benzoquinone doesn’t have the kind of household name recognition of bleach or vinegar, but it plays a big role in research labs and some chemical industries. With a bright color and a tendency to stain skin and surfaces, it looks harmless at first. My early days in the lab taught me otherwise. A small spill stained my glove orange in seconds and left a sharp smell in the air. That day I learned: treat these compounds with respect.
People working with benzoquinone derivatives shouldn’t take safety for granted. This compound reacts easily, especially with strong bases or reducing agents. Moisture and light both speed up decomposition, wrecking the purity and causing unpredictable results. On top of that, inhaling dust or letting it reach skin—these aren’t just minor annoyances. The irritation and potential sensitivity build up with repeated handling.
Plastic bags don’t protect against air or water vapor. My old mentor kept samples in tightly sealed amber glass bottles and stressed why. Glass keeps out air, moisture, and light. Amber blocks out UV rays, which otherwise accelerate breakdown. I’ve seen labs save thousands in wasted material by sticking to these basics.
Every bottle should have a legible label. Marking the date on new stock makes it easier to track shelf-life, which rarely extends beyond a year for this kind of compound. From first-day students to tenured staff, everyone needs clear labels—mistakes happen most often with unmarked containers.
Benzoquinone compounds need cool, dry, well-ventilated conditions. Refrigerators set to store chemicals—not for food—keep temperature consistent and lower humidity, which matters more than most people realize. Warm storage or areas with pipe leaks can ruin supplies overnight. Once, a colleague accidentally stored a fresh batch near a fume hood exhaust; the sample degraded almost completely within days, wasting hours of work.
Desiccants are essential for keeping humidity down. Silica gel or molecular sieves tucked inside storage cabinets pull out stray moisture. That step alone avoids clumping and chemical changes long before a bottle ever gets opened. We learned to rebuild our storage lockers with small wire racks and desiccant pans—a fix that paid off in cleaner, longer-lasting stock.
Gloves, goggles, and lab coats aren’t window dressing, especially for this compound. In halogenated solvents, splashing risk increases. Facilities that cut corners on safety gear often gamble with skin rashes and ruined research. Any spill, however small, calls for quick cleanup. Having dedicated spill kits for oxidizers—usually involving inert absorbent and neutralizing powders—makes for quick work without spreading contamination.
Lab safety culture works best when everyone looks out for each other. New staff should see storage and handling demonstrated, not just explained on a checklist. Locking hazardous chemicals in secured cabinets after hours stops unauthorized access and prevents problems from careless curiosity or forgetfulness.
It’s easy to get complacent, especially during busy weeks. Regular checks of storage conditions, fresh desiccant, and reviewing inventory all add up to safer labs and fewer costly losses. Raising standards takes commitment, not extra cash. I’ve watched lab teams reduce waste and improve safety just by sticking to routines, sharing observations, and holding each other to core safety values. Safe storage isn’t complicated—but it always pays off.
Researchers and manufacturers who work with 2,5-Dimethoxy-1,4-Benzoquinone pay attention to purity for one reason: reliability. High-purity materials keep experiments consistent and results credible. This compound, found in both chemical synthesis projects and biological research, typically reaches a purity level of at least 98%. Labs and suppliers invest significant resources in purification because contaminants can shift outcomes and waste valuable time. It is common to see manufacturers provide certificates of analysis with every batch, outlining the purity and any impurities detected. Analytical techniques such as NMR, HPLC, and melting point confirmation act as safeguards, giving scientists a clear picture of what they’re working with.
Some suppliers selectively offer even higher grades when requested, especially if the application is particularly sensitive, such as in intricate organic syntheses or in enzyme reactivity research. The thinking is simple: accuracy drives progress. So does trust, and it's impossible to build that if somebody down the line wakes up to find unwanted byproducts skewing weeks of data.
In reality, rare chemicals like 2,5-Dimethoxy-1,4-Benzoquinone rarely move in bulk. Most scientific teams order small quantities at a time. The most frequent packaging sizes available hover around 1 gram, 5 grams, and 25 grams per bottle. For most bench chemists or graduate students, even a gram goes a long way. Professionals in chemical manufacturing or larger industrial applications occasionally request larger containers, such as 50 grams or 100 grams, but this is less common. Each bottle arrives sealed, often packed with desiccants to keep moisture at bay.
Packaging small molecules securely matters here. Oxidation and moisture might degrade a fine quinone. Those with experience know that any exposure on the bench can spell disaster, both chemically and financially. Most reputable suppliers choose amber glass vials or bottles, reducing light exposure and avoiding plasticizers found in common plastics. I have opened old plastic-capped vials before and found yellow powders turned sticky, good only for the waste container. Glass and solid caps eliminate that risk.
A company’s reputation for delivering quality product rests as much on pure, well-packaged materials as it does on accurate labeling and prompt delivery. Mistakes damage trust. I remember a project delayed for two weeks because a shipment was missing its certificate of analysis. As digital tracking and testing improve, so does peace of mind.
When shopping for 2,5-Dimethoxy-1,4-Benzoquinone, ask for recent batch verification, proof of purity, and specifics on packaging. Suppliers who invest in stability tests and strong customer communication reduce the risk of wasted time. Adding small, tamper-proof seals on bottles, batch tracking numbers, and quick-response customer teams shows respect for researchers and underlines commitment to quality.
In research, certainty about what sits in a bottle can shape months, even years, of work. Handling the basics—purity and practical packaging—right from the start helps build discoveries that last.
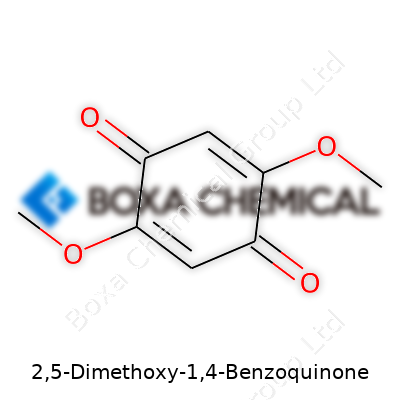
| Names | |
| Preferred IUPAC name | 2,5-Dimethoxycyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˈtuː,faɪv daɪˈmɛθ.əksi wʌn,ˈfɔː bɛnˈzoʊ kwɪˌnoʊn/ |
| Identifiers | |
| CAS Number | 84-86-6 |
| Beilstein Reference | 1721161 |
| ChEBI | CHEBI:15965 |
| ChEMBL | CHEMBL159118 |
| ChemSpider | 11139 |
| DrugBank | DB04268 |
| ECHA InfoCard | 03ff6ec6-1e16-4596-b2cd-634fd7452268 |
| EC Number | 208-952-4 |
| Gmelin Reference | 60713 |
| KEGG | C10714 |
| MeSH | D003827 |
| PubChem CID | 85755 |
| RTECS number | OP4375000 |
| UNII | E8K1O6M52Y |
| UN number | Not classified |
| Properties | |
| Chemical formula | C8H8O4 |
| Molar mass | 168.15 g/mol |
| Appearance | Yellow solid |
| Odor | Odorless |
| Density | 1.42 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.01 |
| Vapor pressure | 3.3 x 10^-4 mmHg (25°C) |
| Acidity (pKa) | 4.08 |
| Basicity (pKb) | 5.08 |
| Magnetic susceptibility (χ) | -73.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.600 |
| Viscosity | 1.2 mPa·s (25 °C) |
| Dipole moment | 2.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 332.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -262.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1840 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 79°C |
| Autoignition temperature | 185 °C |
| Lethal dose or concentration | LD50 (oral, rat): 170 mg/kg |
| LD50 (median dose) | LD50 (rat, oral): 290 mg/kg |
| NIOSH | DS1750000 |
| REL (Recommended) | No REL (Recommended Exposure Limit) established |