Research into benzoquinones stretches back to the earliest days of organic chemistry. Long before anyone thought much about antibiotics, folks in labs started tinkering with simple quinone compounds, watching colors change in glass flasks. As the nineteenth century pushed chemists to isolate new plant toxins and dyes, a handful of scientists stumbled onto derivatives like 2,5-dihydroxy-3-nonyl-1,4-benzoquinone. By the middle of the past century, new extraction and synthesis methods gave researchers better control over these molecules. Interest picked up in the 1960s and 70s, as natural product chemists started identifying similar structures in marine invertebrates. The drive to copy nature’s chemistry set the stage for laboratories to expand and modify base compounds, chasing new medicines, pesticides, and specialty chemicals.
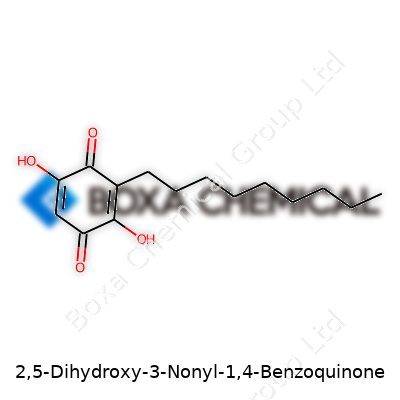
2,5-Dihydroxy-3-nonyl-1,4-benzoquinone comes across as a specialty organic molecule, and for good reason. The presence of a nonyl (nine-carbon) side chain places it in a unique class of substituted benzoquinones. With both phenolic and quinonic functional groups, this compound stands out in redox chemistry and opens unique doors for research into electron transfer. What captured my attention years ago, working with quinones, is how their subtle tweaks (such as the nonyl chain) can dramatically alter their solubility, reactivity, and practical usefulness. The molecule itself remains largely of interest in academic circles, since it's not as easy to come by as other common benzoquinones.
As a solid at room temperature, the compound takes on a distinctive yellow-brown color, typical of quinones but deepened by the extra hydroxy groups. The nonyl side chain, essentially a long hydrocarbon “tail,” pushes solubility toward organic solvents such as diethyl ether and chloroform, whereas water won’t dissolve it much at all. Those two hydroxyl groups on the ring also promise mild acidity, and anyone who's handled similar compounds can confirm their tendency to stain gloves and bench-tops with deep, persistent color. Boiling and melting points run higher than simpler benzoquinones, thanks to the extra mass and chain effects. Pure, well-prepared samples remain stable in sealed containers for years, yet exposure to air or alkalis can trigger slow decomposition.
A good batch of this compound calls for high purity, at least 98% as measured by HPLC or TLC, since trace aromatics and hydrocarbons can skew both analytical and biological tests. Labs require clear, accurate labeling, usually in sealed, inert-glass containers with lot numbers, synthesis date, and storage recommendations noted right on the tag. It takes real discipline to keep a chemical library organized, and mistakes with quinones can lead to ruined experiments or, worse, health risks. The labeling also often lists synonyms and safety details, helping researchers avoid confusion between closely related molecules.
Preparation typically follows an alkylation route, beginning with a protected hydroquinone core. Chemists attach a nonyl group at the third position using a Friedel-Crafts alkylation, followed by careful oxidation to install the 1,4-benzoquinone motif. To restore the hydroxy groups at the 2 and 5 positions, selective reduction and hydrolysis come into play. Each stage of this synthesis brings challenges: overalkylation at the aromatic ring, incomplete oxidation, and degradation under strong acids or bases. It pays to check every round with NMR and chromatography. In my own experience, patience and precise temperature control during oxidation dictate a project’s success or failure.
The molecule’s reactive quinone and phenol groups light up a whole menu of redox and nucleophilic reactions. Alkali can strip a proton from the hydroxyl group, forming anionic species keen to participate in Michael additions or condensation reactions. The nonyl side chain resists alteration, though it boosts lipophilicity, making the core structure easier to work with in organic syntheses. Derivatives appear by adding protective groups, swapping the nonyl for other chains, or reducing the quinone to form hydroquinone analogs. Spectroscopic data confirm site specificity—a crucial detail in pharmaceutical chemistry and toxicity work.
Chemists might call it by its full IUPAC title, but it also appears under names like 3-Nonyl-2,5-dihydroxy-1,4-benzoquinone or Nonylhydroquinone quinone. Each synonym gives clues for literature searches, especially when digging through decades-old journals or patent records. Companies and suppliers put their own spin on naming to distinguish product lines, so vigilance matters to match a bottle’s contents with a project’s needs.
Like most benzoquinones, 2,5-dihydroxy-3-nonyl-1,4-benzoquinone demands respect. The compound can irritate the skin and airway, so gloves, goggles, and a tight-fitting respirator always sit within arm’s reach. Direct contact burns, and chronic exposure to splashes or vapors could damage tissue. MSDS sheets recommend strict fume hood work, rapid cleanup of spills, and double-bagged waste. Transport relies on tightly capped containers, kept cool and out of sunlight—one slip in storage, and the chemical degrades or, even worse, poses a fire hazard.
Quinone chemistry grabs attention in energy research, pharmaceuticals, and agriculture. The electron-shuttling action makes these molecules tempting targets for fuel cell technology. Biochemists study their role in enzyme systems and respiration, while natural product chemists look for leads on anti-tumor and anti-microbial therapies. Recent patents suggest uses in organic semiconductors and redox flow batteries. The hydroxy and nonyl modifications give this compound interesting bioactivity, which draws toxicologists and drug designers to keep probing its potential as a model for nature-inspired drugs.
Active research covers new synthetic routes with greener solvents, catalyst systems, or recycling steps. A growing number of academic groups and startups publish on the use of 2,5-dihydroxy-3-nonyl-1,4-benzoquinone as a platform for derivatization, exploring its interaction with biological systems. I know colleagues who measure enzyme inhibition and antifungal responses, connecting structure-activity relationships for better lead optimization. Analytical chemists build libraries of spectra and chromatographic retention times, helping ensure reliability and reproducibility in every published experiment. R&D also investigates the inclusion of this molecule in next-generation sensors and smart materials.
Ongoing animal and cell culture work aims to pin down acute and chronic toxicity profiles. Exposure to benzoquinone derivatives remains a known risk, with reports of oxidative stress, DNA damage, and tissue necrosis at high doses. Studies track how the addition of a nonyl group tweaks both uptake and clearance in living models. Researchers chase down minimum exposure limits and metabolites, considering links to respiratory and skin disorder rates. It takes detailed, honest reporting to avoid misjudging risk, and most labs keep records on every adverse effect or accidental spill—no cutting corners when health is on the line.
Interest in 2,5-dihydroxy-3-nonyl-1,4-benzoquinone keeps rising as interdisciplinary teams look for cleaner energy sources and nature-derived medicines. Advances in synthetic biology suggest this compound could become a building block for new antibiotics or enzyme mimics. The push for sustainable chemistry means next-generation processes may cut waste and use fewer harmful reagents. Data sharing and open science platforms help younger researchers jump into quinone studies without decades of background reading. As chemical safety standards grow stricter, new methods for detoxification and waste handling will play a much bigger role in industry and academia. The molecule stands as both a scientific challenge and a potential tool for tomorrow’s technology, earning its keep in labs around the world.
2,5-Dihydroxy-3-Nonyl-1,4-Benzoquinone sounds like mouthful chemistry, but it shows up regularly in scientific discussions. This compound falls under the category of quinones—chemicals built around a benzene ring with pairs of carbonyl groups. If you’ve spent time in a chemistry classroom or research lab, the benzoquinone core starts to look pretty familiar. Derivatives like this one don’t just sit in a drawer; real research and industry draw them out for all kinds of practical reasons.
This isn’t just a synthetically cooked-up product. 2,5-Dihydroxy-3-Nonyl-1,4-Benzoquinone pops up in certain plants and fungi. The thing that stands out, especially for biologists, is its role as a natural pigment and, more importantly, as an electron carrier. These factors play straight into its bioactivity and—no surprise—draw scientific attention to its next-level antioxidant and antimicrobial effects.
I first came across discussions about this compound in environmental chemistry research circles. Scientists look for compounds in nature that fight off bacteria, fungi, or oxidative stress, and this one makes the shortlist. We could use more natural answers in a world scrambling with antibiotic resistance and mounting pollution.
The real draw lies in how this compound ends up at the center of health science and industry applications. It’s not as widely known as vitamin C or resveratrol, but it commands respect in the antioxidant game. Researchers value this molecule for its capacity to neutralize free radicals. I’ve seen several papers outlining its ability to protect living cells against damage triggered by unstable molecules—a process that underpins problems ranging from chronic inflammation to some kinds of cancer.
Some labs tap into its natural antimicrobial properties. Its chemical structure disrupts certain bacterial cell walls, making it a candidate for fighting infections. While classic antibiotics tend to overshadow it in drug stores, the push for natural antimicrobials in cosmetics and food preservation brings it forward again. I remember reading about small companies trying to swap out synthetic preservatives for benzoquinone derivatives because consumers showed more trust in nature-based ingredients.
The story doesn’t end with health and wellness. Quinones, and this compound in particular, often land on the materials scientist’s bench for another reason: they take part in electron transfer. This quality puts them to work in technological fields, including organic electronics and the development of new battery types. Research groups worldwide test them to improve energy storage and conversion, hoping for more sustainable and efficient solutions. They aren’t mainstream yet, but patents and university projects keep stacking up.
A lot of research remains stuck in the lab. Scaling the extraction of this compound from natural sources gets expensive. Synthesis racks up costs too, and regulators keep a close eye on safety and potential toxicity. That being said, small biotech startups keep the hope alive by tinkering with bio-based production techniques—using bacteria or yeast to make target molecules faster and cleaner.
Broader acceptance in health or industrial markets depends on practical issues: reliable sourcing, pricing, and toxicology data. The way forward could lie in more collaborations between universities, industry, and regulators—focusing both on lab-driven innovation and consumer trust.
Compounds such as 2,5-Dihydroxy-3-Nonyl-1,4-Benzoquinone get hidden behind long names and even longer chemical formulas, yet they open up options. From my perspective, the deeper you dig into the chemistry, the more possible solutions you discover for some of today’s pressing health and environmental problems.
Handling chemicals in any setting—whether that’s a high school lab, university research space, or industrial site—demands real attention. One story still sits with me from back in college: someone reached to adjust a Bunsen burner without gloves and spent a semester dealing with blisters. Safety mistakes don’t just leave a mark on your hand; they stick in your mind. That sort of lesson goes beyond textbooks. People learn quickly how a casual moment with a toxic or reactive substance can upend their health and their work.
Nothing beats the basics: goggles, gloves, a lab coat, and solid shoes. Eyes will take the brunt of a spill in most accidents, so leaving goggles off the face isn’t worth the risk no matter how quick the task. Nitrile gloves usually handle the job for most chemicals, but strong acids or organic solvents call for materials like neoprene or butyl. Checking the glove compatibility chart posted on the wall isn’t dramatic—it’s practical. Open-toed shoes or sandals have no place in these spaces, as broken glass seems to find them every time.
Some compounds send off strong fumes that drift before anyone notices. Fume hoods really aren’t just a fancy answer to regular fans—they pull vapors and dust away from people’s faces. I’ve seen what happens when someone pours ammonia too quickly outside the hood: coughing fits, watery eyes, and a moment where everyone thinks about quitting. Always run the hood, close the sash as much as possible, and don’t crowd the work area.
Over time, bottles look alike or labels fade. I’ve opened cabinets stuffed with containers and seen more than one bottle labeled with a marker so faint the name barely sticks. Mixing up similar-looking reagents can mean unwanted reactions. Take a minute to check, re-label clearly, and never leave a mess for the next person. The right name on a bottle saves time and prevents accidents nobody needs.
Recipes in chemistry don’t work like they do in a kitchen. Combining bleach with acid, for instance, sends chlorine gas into the air—a hospital trip waiting to happen. Before adding anything together, double-check reliable sources or a detailed chemical compatibility chart. Even a basic lab manual or manufacturer’s guide will save you from a headache later.
The best labs I’ve seen keep sinks, eyewashes, and emergency showers unblocked and obvious. It’s tempting to pile boxes near them, but doing so slows escape and clean-up if a spill happens. Know the nearest exits, wash locations, and practice what you’ll do in a real emergency. People freeze up in the moment unless they’ve done a walk-through before.
No system works if people don’t speak up about things that almost went wrong. Sharing stories about near misses in the break room helps build a sense of teamwork and reminds everyone what’s at stake. Sometimes the only real difference between a scare and a disaster is a few inches or seconds.
Lab safety boils down to mutual awareness. Respecting the space and the people in it means everybody gets to go home healthy. It only takes a moment of carelessness for something to go sideways, but steady focus and good habits stack up, day by day.
Every chemical comes with its own quirks. Some break down when moisture sneaks in, others react if left exposed to light or warmth for too long. Back in my early lab days, I learned the hard way that even simple sodium hypochlorite starts to lose strength after a few weeks if the cap’s not screwed on tight. The world doesn’t give you a free pass—oxygen, sunlight, and heat are always working to spoil a good batch.
People working in research or industry know the risk of using a degraded product. If a compound goes bad, your results go out the window, and money goes down the drain buying replacements. Benzene rings don’t just disappear, but other chemicals love to rearrange themselves all too easily if the conditions nudge them. Anyone handling chemicals—nurses in a hospital storeroom, students in a university lab, a technician on a production floor—knows a product only performs well if it stays the way it started.
Temperature has a big say in whether a product keeps or not. Acetonitrile, for example, sits comfortably in a cool, dry place, but bring the heat up and you risk losing it to evaporation or even fire. There are stories of solvents stored near windows that evaporate just from getting too warm in the sun during a long weekend. Even less volatile materials can absorb water from the air and start clumping or degrading—magnesium chloride draws in water so fast you can almost watch the crystals turn to soup.
If a chemical container feels damp or crusty, you know it’s lost its edge. I’ve seen reactions fail in student labs because a bottle sat open for just a week, absorbing moisture it shouldn’t. Keeping chemicals dry is not just a technicality—it’s a rule most ignore only once.
A good storage container can make as much difference as a good lock on your front door. Brown glass bottles shield sensitive chemicals from light, cutting down on reactions triggered by UV rays. Tight screw caps or rubber seals keep out air and water. Plastic has its place, but glass remains king for acids and solvents that might chew through other materials. I once saw a bottle of nitric acid eat through a plastic lid in a single summer, a lesson that stuck with me.
Labels sometimes seem like a small detail, but they’re vital. Ink that fades or smears leads people to guess and mistakes happen. Proper labeling with storage date, strength, and hazard symbols tells the next person exactly what’s inside, so nobody plays guessing games with safety.
Safety officers in regulated environments track every chemical batch. They don’t just check expiry dates—they run weekly or monthly audits. Chemicals past their prime get removed for proper disposal. Home labs and schools can learn from this habit. Keeping an inventory saves money, keeps everyone safe, and prevents accidental misuse. In my experience, a shelf with a clear system—oldest at the front, new deliveries at the back—cuts down on mistakes and keeps people honest about what needs replacing.
No matter the product—solvents, salts, or bio-reagents—stable storage matters. A dry, cool, well-ventilated space can often double the usable life of a compound. Taking the time to set things up right pays back every single day.
2,5-Dihydroxy-3-Nonyl-1,4-Benzoquinone isn’t a chemical that pops up every day in the mainstream. This compound often attracts attention in environmental research, especially in relation to its marine origins. Its structure puts it among quinones, a group of compounds scientists watch closely for their potential biological activities and toxicities. With that in mind, it’s worth asking—what health or environmental risks emerge from this compound?
Research on 2,5-Dihydroxy-3-Nonyl-1,4-Benzoquinone centers mostly around its role in marine organisms. Quinones in general come up in studies on oxidative stress and cellular toxicity. Some of them cause irritation or trigger allergic reactions after skin exposure. There’s evidence from animal studies on similar compounds that suggests quinones can generate reactive oxygen species, resulting in cell damage. If a compound keeps prodding cells this way, long-term exposure could have negative effects for people working with it, especially in poorly ventilated spaces without protective gear.
Breathing fine dust or vapors is a real risk in any chemical lab. Most chemists respect quinones by using gloves, goggles, and fume hoods. My own work with dusty compounds drives home the need for real caution. I’ve seen what careless handling does to a person’s skin—redness, peeling, or even blistering with repeated contact. It’s better to treat every unknown with the seriousness it deserves.
Out in the environment, quinones hang around longer than you’d hope. In soil and water, they break down slowly unless microbes step in. Some benzoquinones act as antimicrobial agents, so they make life harder for those helpful bacteria. This slows down natural breakdown and can disrupt local ecosystems. You won’t find mountains of data on 2,5-Dihydroxy-3-Nonyl-1,4-Benzoquinone polluting rivers or drinking water, but the close relatives in its family point to some risks if improper disposal or accidental spills turn up. It’s easy to imagine a scenario where industrial or research sites forget the warning signs and dump their waste without following proper guidelines. That neglect seeps down to groundwater and works its way up the food chain.
Most chemicals come with risks that amplify when we ignore rules or cut corners. I’ve worked in labs where the safety data sheet collects dust, and I’ve worked in places where every researcher undergoes yearly retraining. Consistent education goes a long way. Every person who comes into contact with a new substance should read up on its hazards, wear the right protective gear, and know what to do in case of spills or exposure.
Disposal practices often fall short of ideal. Neutralization, incineration under controlled conditions, and strict labeling keep toxic quinones out of the environment. Governments should keep pushing for regular monitoring of water around factories dealing in hazardous organic compounds.
Working safely with any compound, particularly quinones, starts with respect for the unpredictability of chemistry. Even with limited studies, similar molecules have left a strong enough paper trail—one that should not be ignored in the rush for productivity or convenience. Chemical breakthroughs only mean something if people and the planet don’t pay the price later.
Purity isn’t something you think about every day, but the difference between 96%, 98%, and 99% purity can shape how a product is used and what it costs. I’ve watched researchers go out of their way for even one more percentage point of purity—sometimes for good reason. In pharmaceuticals, for example, trace contaminants can cause side effects or throw off test results. For folks in electronics and laboratory research, just a fraction of a percent can change how components function or experiments turn out.
The most common purity grades you see on the market line up with technical, laboratory, food, and pharmaceutical standards. Technical grade lands around 96-98%. It works fine for industrial cleaning, water treatment, or chemical synthesis where a little impurity isn’t a deal breaker. In the food sector, 99% and up becomes the expected baseline to avoid off-flavors, textures, or health issues. Labs and pharmaceutical companies will stretch for 99.5% or even “analytical grade” that’s been through extra purification steps.
As a buyer, the advertised number isn’t everything. I’ve heard stories from chemists who ran identical procedures with different suppliers’ 99% white powders—and got different results. That extra one percent should be made of well-documented, non-toxic compounds, not mystery leftovers. For buyers wanting to make a responsible decision, always review the certificate of analysis and safety information, no matter how sparkly the purity claim looks on paper.
Packaging sometimes gets less attention than it deserves. It’s easy to overlook, but it can make the difference between easy storage, safe handling, and a messy, expensive accident. Most chemicals and powders with higher purity fit into a few standard packaging types. Smaller lots, say 100 grams up to a few kilograms, arrive sealed in plastic, glass, or metal containers. These are tightly closed to prevent contamination and moisture. It isn’t just about presentation; purity drops quickly if compounds sit around uncapped or exposed to air, humidity, or heat.
Large-scale users—factories, big laboratories, or farms—usually order materials in drums, sacks, or intermediate bulk containers (IBCs). These heavy-duty containers can move with forklifts and sometimes use special plastic liners to keep out air. For dangerous or highly reactive chemicals, I’ve seen companies double-wrap or use thick-walled drums. Safety, here, beats convenience, and it can’t be stressed enough after seeing what even a small spill can do to a workspace. In the past, I’ve handled materials that arrived in clumsy, cheap bags that nearly split open—definitely not worth saving a few dollars if you end up losing half the shipment.
The choice between bulk and small packaging usually comes down to how quickly you’ll use the product and whether you have a safe way to store leftovers. Higher purity batches cost more and need more care, so don’t order kilograms if you only need a spoonful. For those working with sensitive applications—electronics, food, or medicine—the safest bet is certified, high-purity material in sealed, tamper-evident packaging. Avoiding shortcuts at this stage saves headaches later.
As a practical tip, always ask for detailed documentation: lot numbers, manufacture dates, and certificates. Suppliers worth partnering with will answer without hesitation. Sustainable packaging is gaining steam, too, so watch for returnable containers or recyclable plastics—both cut down on waste without skimping on safety.
| Names | |
| Preferred IUPAC name | 2,5-dihydroxy-3-nonylcyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˈtuː,faɪv daɪˈhaɪ.drɒk.si θriː ˈnəʊ.nɪl wʌn,fɔː ˈbɛn.zəʊ.kwiː.nəʊn/ |
| Identifiers | |
| CAS Number | 18333-51-2 |
| Beilstein Reference | 2341080 |
| ChEBI | CHEBI:72715 |
| ChEMBL | CHEMBL2007615 |
| ChemSpider | 175434 |
| DrugBank | DB08342 |
| ECHA InfoCard | 03b77aaf-d57c-4ae0-8e86-7e17fbcf0e97 |
| EC Number | 1.6.5.2 |
| Gmelin Reference | 107737 |
| KEGG | C06431 |
| MeSH | D010539 |
| PubChem CID | 65668 |
| RTECS number | OU8225000 |
| UNII | 1833F8V74N |
| UN number | NA-UN2546 |
| CompTox Dashboard (EPA) | EPA CompTox Dashboard: CHEMBL187711 |
| Properties | |
| Chemical formula | C15H22O4 |
| Molar mass | 280.39 g/mol |
| Appearance | Yellow solid |
| Odor | Odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.72 |
| Vapor pressure | 2.78E-7 mmHg at 25°C |
| Acidity (pKa) | 10.0 |
| Basicity (pKb) | pKb = 4.69 |
| Magnetic susceptibility (χ) | -75.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.587 |
| Viscosity | Oil |
| Dipole moment | 5.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 577.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –528.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1202.7 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | C01EB18 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P280, P261, P264, P271, P304+P340, P312, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: >100°C |
| LD50 (median dose) | LD50 (median dose): LD50 oral (rat) > 2000 mg/kg |
| NIOSH | DJ8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |