Chemists in the early twentieth century started looking closely at benzoquinone derivatives. In research literature from the 1920s, 2,5-dihydroxy-1,4-benzoquinone sparked curiosity thanks to its unique redox activity. Scientists had begun to understand the patterns behind hydroxyquinones, putting this molecule on the radar for battery chemists and dye manufacturers. By the postwar era, analytical advances revealed its structure and the impact of hydroxy substitution. Scientists shared protocols for its preparation, identified its tautomers, and added it to the toolkit for chemo-informatics before that term existed.
2,5-Dihydroxy-1,4-benzoquinone appears as a yellow-brown crystal or powder with a smoky, slightly acrid odor that makes it stand out in labs. With potential as a building block for electrodes, dyes, and sensors, this compound drew attention for efficiency in proton conductivity and its ability to be modified. Availability from chemical suppliers stays steady, both for small-scale research and commercial batch orders.
With a melting point in the range of 285-290°C, 2,5-dihydroxy-1,4-benzoquinone resists thermal breakdown longer than many simple quinones. Solubility tilts toward the polar: it dissolves in water, ethanol, and hot acetic acid. The distinct color signals its redox properties, toggling between oxidized and reduced states as needed. This behavior ties directly to its pKa values, which reflect the relatively acidic protons of hydroxyl groups, making it more reactive around bases or nucleophiles.
Chemicals at this level need clear labeling due to their reactivity and research relevance. Purity often sits above 97%. Main packaging stays dry and light-protected, in glass or polyethylene—anything else can trigger partial reduction or contamination. Suppliers print shelf life, batch number, and protein content for uses where contaminants throw off calibration curves, as in electrochemistry.
Making 2,5-dihydroxy-1,4-benzoquinone on a lab scale starts from hydroquinone or para-benzoquinone, then uses oxidizing agents such as ferric chloride, silver oxide, or nitric acid under controlled conditions. Cold solutions restrain over-oxidation, while filtration and controlled recrystallization help isolate the target molecule. Traces of residual oxidants always complicate purification, so repeated washes with water and ethanol become standard steps.
The molecule thrives as a substrate in oxidation-reduction cycles. Researchers commonly use it in redox titrations or as a mediator in electrochemical experiments. The ortho-hydroxy patterns open doors to functionalization: methylation, halogenation, or even slow stepwise polymerization. As a chelator, it binds with transition metal ions, forging paths toward metal-organic frameworks and enzyme mimics.
The rich synonym pool helps locate 2,5-dihydroxy-1,4-benzoquinone in any catalog: people call it 2,5-DHBQ, duroquinone, quinizarin, or p-benzoquinone-2,5-diol. Chemists sometimes refer to it by the systematic name, but manufacturers and product catalogs stick with the short form for clarity.
Toxic dust from quinone derivatives remains a key lab concern. Exposure causes irritation to skin, eyes, and lungs. Safety data sheets call for gloves, goggles, and strong ventilation. No open flames or heat since this stuff decomposes and releases hazardous fumes under strong thermal stress. Emergency showers and eyewash stations sit nearby in every lab working with hydroxybenzoquinones, and container disposal stays in line with regional chemical waste protocols.
2,5-Dihydroxy-1,4-benzoquinone serves researchers in energy storage—its participation in rechargeable proton batteries shows promise for affordable, flexible grid storage. Dye chemists appreciate its intermediate role in the creation of anthraquinone-based dyes, while biochemists use it to probe electron transport and oxygen reactivity for enzymes. The molecule’s ability to reversibly accept and donate electrons keeps it relevant in redox flow battery development, molecular electronics, and as a ligand scaffold for catalytic reactions.
In recent years, research teams have unlocked new polymeric forms to maximize surface area and conductivity. This led to prototype battery electrodes outpacing some commercial organic cathodes. Coordination chemists use it as a bridge for assembling metallo-organic structures with tailored electrical properties. Innovation doesn’t stop there: it features in studies on radical scavenging and shows up as a reporter in bio-assays for oxidase enzymes. Advances in spectroscopic and X-ray techniques let people map its interaction at the atomic level, deepening understanding every year.
Animal model studies show that high-level exposure leads to acute toxicity with oxidative stress, but strict handling cuts risk dramatically. Realistically, exposure in normal lab or industrial practice stays low. Regulatory bodies classify this compound as an irritant, not a carcinogen. Ongoing studies watch for chronic low-dose effects on DNA and proteins, pushing labs to implement full containment in manufacturing and bulk applications.
Industrial chemists have high hopes for 2,5-dihydroxy-1,4-benzoquinone in new energy frameworks, where its low environmental impact and cheap synthesis beat out precious-metal-based competitors. As grid-scale batteries demand safer and recyclable components, its use in aqueous flow batteries could deliver high-capacity storage at dramatically lower cost. Chemistry educators explore it for teaching redox and ligand chemistry. If molecular customizations keep improving, this little quinone might anchor future materials for sensors, catalysis, and biocompatible electronics.
In the huge world of chemicals, 2,5-Dihydroxy-1,4-benzoquinone stays mostly under the radar. Usually shortened as DHBQ, this small molecule grabs a scientist’s attention because of its two hydroxyl groups and a quinone core. Whenever a textbook shows off a reaction involving ring structures and electron shuffling, a compound like this gets a line or two—but its story stretches further than dry chemistry pages might suggest.
Scientists digging for safer and sustainable energy storage got extra excited after testing DHBQ in organic battery research. In these experiments, DHBQ acts as a charge carrier inside a redox flow battery—basically, it stores and releases electricity as ions move between two tanks. Most commercial batteries count on heavy metals, leading to price spikes and supply chain drama. DHBQ and other organics don’t hang onto those problems. Cost stays lower, materials can be sourced closer to home, and the working lifespan of these molecules often reaches impressive lengths.
A 2020 study from the Journal of Power Sources highlighted DHBQ’s redox stability and fast electron transfer rate. That points to a real chance to improve grid-scale battery performance. Places left without reliable electricity, or trying to cut back on fossil fuel use, stand to benefit a lot here. As a kid, we experienced blackouts that kept us sweating through the Texas summer. If researchers keep fine-tuning these organic systems, future homes might keep the lights on with chemistry like DHBQ instead of lithium or cobalt.
Industries making inks and dyes have turned to DHBQ’s quinone structure for specific reactions that yield bold colors. Different substitutions on the aromatic ring give rise to shades that textile producers or paint manufacturers find valuable. Over the years, stricter regulations around toxic heavy metals in pigments pushed companies to explore safer molecules. DHBQ offers a workaround—its breakdown products tend to be less harmful, and third-party tests usually check out for lower environmental impact compared with many older alternatives.
Medicinal chemists often start their search for new drugs with ring systems like those in DHBQ. Its oxygen-rich structure makes it a building block for synthesizing complex molecules, including potential antivirals or cancer treatments. For example, a 2019 Bioorganic & Medicinal Chemistry study reported successful use of DHBQ as a scaffold in experimental inhibitors targeting certain enzymes in bacteria. Trial and error remains slow, but this sort of research lays the foundation for routes that lead to new antibiotics or antimalarial drugs.
Chemists also tap DHBQ’s potential as a catalyst or co-catalyst. In organic labs, it participates in oxidation and reduction reactions, moving electrons with reliability across different environments. Students in classrooms across the country use DHBQ as part of training in practical synthesis. Whenever a compound helps teach the basics in an affordable and safe way, it holds its value over the years.
The “green chemistry” movement emphasizes less hazardous materials, renewable feedstocks, and energy-saving processes. Since DHBQ can be prepared from plant-based sources, its supply doesn’t have to depend on petrochemicals forever. Life cycle analysis studies published over the past decade suggested that, with investment in biotechnological methods, DHBQ production could shrink its carbon footprint. My own faculty advisors often repeated the importance of finding chemistry that works in water under mild conditions, and DHBQ stands out for its adaptability here. The more work that goes into sustainable sourcing, the more promising its applications look for clean-tech companies chasing lower emissions and better waste management.
2,5-Dihydroxy-1,4-benzoquinone falls into the quinone family, a group known for their bright yellow and red pigments and crucial roles in biological systems. This molecule looks simple at first glance, but a closer look reveals a surprising amount of chemistry packed into its small structure.
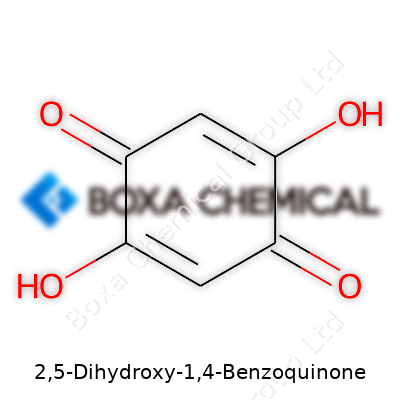
The molecular formula is C6H4O4. That gives you six carbon atoms, four hydrogens, and four oxygens. These atoms arrange themselves as a six-carbon ring, much like benzene, but two of the ring’s carbons each hold an oxygen as a double bond (these are the quinone oxygens at the 1 and 4 position). Two more carbons each hold their own hydroxyl group — one at position 2, the other at position 5. Both -OH groups attach opposite each other on the ring, which affects the molecule’s stability and its ability to make hydrogen bonds.
Draw it out, and you see the pattern: O= at carbons 1 and 4 (the two double-bonded carbonyl oxygens that define the "quinone" part), -OH at carbons 2 and 5 (where those dihydroxy groups land), and simple hydrogens filling in the rest. Such a structure means the molecule's planar, holding its shape with a flat ring, helping it stack up with other flat molecules like the classic examples in crystalline pigments.
Back in my lab days, I watched these molecules play key roles in how batteries shuttle electrons back and forth. The 1,4-benzoquinone core always impressed me because of its role as an electron acceptor. Add in the dihydroxy groups, and you unlock new chemical possibilities – more electron-donating power, more solubility, and new hydrogen-bonding tricks. In environmental science, this molecule pops up in natural humic substances and soil organic matter. People sometimes underestimate those humble-sounding organic acids – their presence shapes how metals move through water and soil, how nutrients cycle, and how polluting toxins bind or break down.
Humans never stopped searching for color, either. Quinone derivatives, including the dihydroxy variants, gave the world vivid dyes and inks stretching back to the earliest fiber crafts. Natural chemistries like these inspired ways to create non-toxic, renewable colorants for food and textiles. That has stayed on my mind since working with toxic pigments in art restoration – knowing there’s a safer path possible, thanks to molecular design.
People run into trouble when they try scaling up production without thinking about environmental impact. Regular production produces waste. Some of the solvents and reagents needed for older syntheses of benzoquinone derivatives are harmful themselves. Factories and research labs can turn to cleaner, greener synthetic routes. Biocatalysis, for instance, relies on enzymes and typically runs at lower temperatures with less toxic leftovers. Universities and industry could channel funding into these research areas. Regulations should tighten up on waste and emissions, but practical support for greener chemistry makes real change happen faster.
Teaching about basic molecules like this quinone early in chemistry education can drive curiosity. Students pick up not just facts on formulas and molecular geometry, but practical context for later careers. Cheaper, cleaner synthesis paired with strong chemical education paves the way for less hazardous products down the line – in batteries, agriculture, dyes, and water treatment.
2,5-Dihydroxy-1,4-Benzoquinone often turns up in labs where researchers look for new ways to harness redox chemistry. Back in my grad school days, few things grabbed my attention like safe storage, especially with compounds that leave no margin for error. I learned early that a tightly sealed bottle often makes the difference between a routine day and an emergency.
Left uncovered in humid air, 2,5-Dihydroxy-1,4-Benzoquinone starts soaking up water from its surroundings. Pretty soon it clumps, degrades, and loses potency. That’s not just a waste of reagent money—it's a waste of everyone’s time. Labs fixing their eye on best practices stick this stuff in containers that close tight and resist corrosion. Glass bottles with PTFE-lined caps keep the compound dry and safe. Toss in a silica gel packet for good measure. Folks trust these bottles more than plastic since the material lasts longer and stays clean.
Heat and UV light cause chemicals like this to decompose, sometimes with nasty byproducts. Basic room temperature storage works unless a fluke heat wave shows up. Always stash these in a dry, cool cabinet out of sunlight. I’ve rubbed elbows with too many scientists who've had entire sample collections ruined on a sunny windowsill. Keep cabinets locked away from direct light and far from radiators, autoclaves, or anything else throwing off extra warmth.
Getting complacent leads to hospital visits. In my experience, gloves rarely fail unless someone tries to stretch their use to save money. Nitrile gloves and safety goggles never go out of style. Chemical splash goggles beat regular glasses every day, since fine dust from quinones feels nasty in your eyes and can leave burns. Always work in a fume hood. These chemicals let off fumes that irritate nose and throat before you know it. I’ve watched too many folks skip the hood, then blame everything but themselves for headaches and sinus flare-ups.
No one wants bench stains or chemical burns on their hands. A simple spill kit with absorbent pads, disposal bags, and detergent handles most bench-top mishaps. I once knocked over a small beaker and watched the red stuff creep toward my notebook. Wiping up with a dry towel pushed the dust everywhere. Only soapy water and a fresh pad kept the mess from spreading further. Folks dump waste in tightly sealed, labeled containers—never down the sink. Your building’s safety manager can show you the chemical waste route. Trust me, following those steps beats the environmental fines that have hit a few labs I’ve worked in.
No high-end glove box or temperature sensor replaces solid training. I’ve seen new hires triple-check the storage protocols, while seasoned folks sometimes forget the basics. Those with the best safety records quiz themselves on chemical behaviors, watch out for unexpected clumping or color changes, and label everything like their job depends on it.
Staying sharp and following strict guidelines saves lives, reputations, and budgets. Every lab has its quirks, but nobody gets to ignore fundamentals when dealing with quinones. If you’re not sure, ask someone. Most of us would rather answer “dumb” questions than get a call from HR after a safety incident.
Working in a lab means running into plenty of chemicals with complex names, but every bottle brings its own type of trouble. 2,5-Dihydroxy-1,4-Benzoquinone, for example, has a sharp story. This compound might not make headlines like cyanide, but it commands respect. Even working briefly near it leaves you with a new appreciation for what can go wrong fast.
The first lesson from direct experience: do not underestimate powders and crystals that stain. The fine dust from this compound drifts on gloves, sticks to surfaces, and dishes out toxic effects. If you breathe it in or somehow get it on skin, irritation follows. Mild cases mean coughing or itchy skin, worse cases can escalate. Getting it in your eyes rushes you straight to the eyewash station, and nobody wants that.
People sometimes joke that it’s the “ones you can’t pronounce” that bite you hardest, and 2,5-Dihydroxy-1,4-Benzoquinone fits that bill. It’s toxic if swallowed, inhaled, or brought into contact with skin. Many quinones, including this one, cause respiratory and nervous system problems after repeated exposure. Chronic exposure links directly to damage of internal organs in animal studies.
The dust settles everywhere. In a warm, dry lab, static lifts granules off the bench and floats them into instruments, sleeves, and masks. I remember an incident where someone shook open a bag too fast, sending a faint cloud across the bench—not a hospital trip, but sharp coughing and red eyes for hours. No one needs that kind of scare. Proper chemical storage, marked containers, and having spill kits within reach helps, yet personal habits matter most.
Personal protective equipment changes the whole game. Nitrile gloves, safety goggles, and lab coats help trap any wandering dust. Face shields and dust masks give more assurance during weighing or mixing. I’ve learned to double-glove and check the seal on masks, especially when using spatulas around fine powders.
Ventilation makes a huge difference. Pulling fumes away with a working fume hood lowers risk. That means no shortcuts, even for a quick sample. Keeping chemicals capped, handling small amounts, and labeling every flask keeps accidental exposure low. Benches stay cleaner when you plan out steps before even touching the bottle.
Nobody wants to scramble when a spill happens. Protocols matter—cleanup kits with absorbent pads, neutralizing powders, and sturdy containers sit ready. Washing up straight after work with plenty of water, not just a quick rinse, avoids surprises from missed residue.
Some people scoff at caution, but the stories from seasoned lab hands tell a different tale. I’ve seen productivity crater for days after one careless error—benches cordoned off, paperwork piling up, bad air in the lab. The culture of safety means caring about colleagues, too. If your coworker gets exposed, everybody deals with the fallout. Training and real stories have more impact than posted signs.
Mistakes with chemicals like 2,5-Dihydroxy-1,4-Benzoquinone ripple far: ruined batches, lost time, real human risk. Respect, preparation, and clear communication set the tone. I learned early from more experienced chemists—nothing replaces being ready, every time you uncap something with a name like this.
Tracking down a chemical like 2,5-Dihydroxy-1,4-Benzoquinone (also known as p-Benzoquinone Dihydroxy) shows that research labs and manufacturing teams never have it easy. It’s one of those specialized compounds—used mostly in organic synthesis and material science applications—that’s not exactly sitting on retail shelves. The hunt begins online, and quality matters as much as the source itself. Genuine suppliers list purity specs, material safety data, and clear contact lines. Jumping at the first result without seeing proper documentation can lead to costly delays, contaminated product, or headaches with compliance.
Many suppliers focus on academic and industrial research. Trusted global names like Sigma-Aldrich, Alfa Aesar, and TCI America regularly list 2,5-Dihydroxy-1,4-Benzoquinone. They screen their customers, often requiring proof of institutional use or business licensing. Smaller chemical companies often appear with tempting prices. It makes sense to double check reputation—look up third-party reviews or speak directly with customer service before forwarding payment details.
Chemical packaging isn’t just a detail for safety officers—it changes daily routines in the lab. Common sizes range from a few grams in glass bottles (perfect for academic work, method development, or testing) up through 25-gram or 100-gram jars for projects that need scale or repeat syntheses. Bulk purchases for industrial work sometimes appear in larger containers, typically amber glass or HDPE plastic to fight off light or moisture. That matters. Light and moisture cause degradation for a lot of finely-tuned organic molecules.
I’ve learned, working benchside, never to skimp on packaging even if the price difference feels steep. Unsealed or poorly-sealed powders can solidify, clump, or spoil. That ruins the entire experiment and burns through budgets. Amber bottles with tight closures preserve function much longer, so the expense also means fewer returns and less downtime for reordering.
Losing track of chemical safety guidance creates bigger risks than experimental failure. Reputable suppliers provide full Safety Data Sheets (SDS) and shipping guidelines. Transport of 2,5-Dihydroxy-1,4-Benzoquinone has to follow regional and international regulations, including those touching hazardous material. Domestic vendors ship using insured couriers and specialized packing (sometimes including extra absorbent material or secondary containment if needed). International orders require import/export documentation and certifications of analysis.
Connecting with a supplier’s application scientist or technical support team always pays off. They help clarify which batch size, bottle type, and purity level lines up with your plans. This limits waste from product that’s ill-suited for the intended work. In my experience, reaching out directly—even by old-fashioned phone call—and building rapport with a vendor’s technical team speeds things up versus bouncing emails or web forms.
People who pool their orders with nearby departments or research colleagues can sometimes access discounted rates, especially if buying off contract or for common research themes. It reduces shipping impact and packaging waste, cutting down on costs and headaches for everyone.
When precision, traceability, and safety drive chemical purchasing, a little personal diligence brings long-term peace of mind. Chasing the right supplier and the best-suited packaging for 2,5-Dihydroxy-1,4-Benzoquinone means smoother research, fewer disruptions, and reliable results—every single batch.
| Names | |
| Preferred IUPAC name | 2,5-dihydroxycyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˌdaɪ.haɪˈdrɒk.si wʌn ənd ˈfɔːr ˌbɛn.zəʊ.kwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 615-94-1 |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:18151 |
| ChEMBL | CHEMBL16137 |
| ChemSpider | 5017 |
| DrugBank | DB04250 |
| ECHA InfoCard | 03b1e3ba-6d2d-487b-b240-bfbcf4c6f693 |
| EC Number | 204-616-7 |
| Gmelin Reference | 166133 |
| KEGG | C00951 |
| MeSH | D003856 |
| PubChem CID | 8650 |
| RTECS number | DO3150000 |
| UNII | 1A5P837FZH |
| UN number | Not classified |
| Properties | |
| Chemical formula | C6H4O4 |
| Molar mass | 126.11 g/mol |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 1.596 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.54 |
| Vapor pressure | 1.25E-6 mmHg at 25 °C |
| Acidity (pKa) | 2.58 |
| Basicity (pKb) | 7.46 |
| Magnetic susceptibility (χ) | -72.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.774 |
| Viscosity | 100 mPa·s (20 °C) |
| Dipole moment | 2.7129 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 149.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -404.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -653.7 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P273, P301+P312, P304+P340, P312, P330, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2,2,0 |
| Flash point | 185 °C |
| Autoignition temperature | Autoignition temperature: 410 °C (770 °F; 683 K) |
| Lethal dose or concentration | LD50 (Oral, Rat): 320 mg/kg |
| LD50 (median dose) | LD50 (median dose): 400 mg/kg (rat, oral) |
| NIOSH | DJ4800000 |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 10 mg/m3 |