Once upon a time in early 20th-century chemistry labs, scientists noticed that tweaking the quinone ring with chlorine produced unexpected effects. 2,5-Dichloro-1,4-Benzoquinone, sometimes compared to its more famous cousin p-benzoquinone, soon earned its place thanks to its role as an intermediate in dye synthesis. During the massive post-war industrial boom, dye manufacturers worldwide wanted sharper colors and greater dye fixation, which led them to this compound. Its path connects the dots from German coal tar chemistry traditions to modern organic electronics, not just because it colors things but because it quietly enables a whole network of reactions in specialty manufacturing.
My first encounter with 2,5-Dichloro-1,4-Benzoquinone happened while hunting for an oxidant in a research-grade organic synthesis. It shows up as a pale yellow powder with a sharp, biting odor and a tendency to irritate the eyes and nose. Factories churn it out in large batches; those yellow drums bear the weight of many downstream products, from pharmaceuticals to agrochemical formulations. Chemists value it for its high oxidation potential and ease of handling. The market treats it as a specialty chemical, with production volumes that remain modest compared to bulk feedstocks, but enough to maintain regular trade across laboratories and small factories worldwide.
This compound, with a molecular formula C6Cl2O2, forms yellow crystalline solids that melt around 107 to 109°C. It dissolves surprisingly well in organic solvents such as acetone and benzene but stubbornly refuses to interact much with water. On the bench top, its volatility stays low, so it tends not to evaporate rapidly or escape into the air when handled at room temperature. Chemically, it acts as a strong oxidant, picking up electrons and facilitating transformations that few other handy organics will tackle. Its chlorinated structure enhances its reactivity relative to simple benzoquinones, a feature valued in both synthetic and analysis labs.
A typical lab label will read “2,5-Dichloro-1,4-Benzoquinone, 98%, CAS No. 616-23-9.” Most suppliers guarantee purity above 97% and test for residual water and common halogenated impurities. Firms with a focus on chemical safety print warnings about skin and respiratory irritation in bold letters. On the shipping side, United Nations regulations put this chemical under environment-hazardous labels, especially in bulk quantities, demanding rigorous documentation. The safety data sheets speak to its reactivity and caution about proper storage away from acetic acid, strong bases, and direct sunlight to minimize decomposition and fumes.
The classic route for synthesizing this compound involves direct chlorination of hydroquinone. Most production lines use chlorine gas bubbled through an aqueous or alcoholic hydroquinone solution, under precisely tracked temperatures to ensure the 2,5-substituted product predominates. Gloves, goggles, and ventilated fume hoods always enter the scene here, because both chlorine and the final benzoquinone are harsh on biological tissue. Some labs have experimented with milder oxidants and selective chlorination by using N-chlorosuccinimide, but cost and scale-up complications often push them back to the tried-and-true chlorine gas procedure. Downstream, the crude product receives a solvent recrystallization to boost purity and strip out unwanted byproducts like 2,6-dichloro variants.
2,5-Dichloro-1,4-Benzoquinone presents a flexible platform for further transformation. Researchers can reduce it to the corresponding dihydroquinone under mild hydrogenation or use metal hydrides. Nucleophiles target the aromatic ring with substitutions at the remaining para positions, letting chemists build more elaborate ring systems for dyes and pharmaceuticals. Analytical chemists turn to this molecule when they need to quantitatively oxidize specific analytes, especially in the world of sugar and carbohydrate detection. Modern organometallic labs sometimes harness these quinone scaffolds to support electronic modifications in the development of novel catalysts.
Folks in the industry know this chemical by several aliases: 2,5-DCBQ, Chloranil Yellow, Dichlorobenzoquinone, and even Paraquinone Dichloride. Some catalogues merge it into broader “chlorinated quinones” categories, but careful users recognize the distinctions. Pharmaceutical regulatory filings, especially those in the European Union, ask for the full IUPAC name: “2,5-dichloro-3,6-cyclohexadiene-1,4-dione.” The names matter because mislabeling between positional isomers leads to confusion and, sometimes, ruined experiments.
Nobody should take lightly the irritant power of dichlorobenzoquinones. In our university storerooms, this chemical never sits out for long periods. We always stored it in closed, well-ventilated cabinets. Splash goggles and double nitrile gloves became standard gear when handling even a few grams. The fumes, if inhaled, immediately burn the throat and can provoke coughing fits strong enough to drive a researcher from the room. On the industrial scale, exhaust hoods and robust air monitoring offer peace of mind. OSHA and the EU’s REACH regulations set strict personal exposure limits and classify this quinone as hazardous, so any workflow involving it calls for full risk assessments and safety drills.
Industries count on 2,5-Dichloro-1,4-Benzoquinone as a go-to oxidant and key building block. Textile dye houses rely on it for pre-treating fabrics and synthesizing reactive dyes offering bold color fastness. Pesticide development pipelines use it for producing specific phenolic intermediates. Analytical chemists tap its redox properties for carbohydrate assays, especially in detecting subtle sugar residues in complex mixtures. Laboratories producing electroactive polymers and organic light-emitting diodes list this molecule as an essential precursor, because its resonance structure provides electronic stability and tuning for semiconducting films. Small pilot lines even turn out specialty medicines with halogenated scaffolds that start from this compound.
Recent years ushered in a renewed interest in modified benzoquinones. Green chemistry circles push for less toxic synthesis routes, swapping out chlorine gas for milder, safer oxidizing agents and devising closed-loop processes to capture vented emissions. Analytical groups experiment with immobilized 2,5-DCBQ on silica beads for reusable column chromatography. Others explore this molecule in battery research, seeking stable redox couples for new organic flow battery designs. Startups working on agricultural technology look for ways to graft the quinone nucleus onto existing pesticides or plant growth stimulants, searching for better yield and lower toxicity profiles. These efforts reflect a clear trend: the field invests in innovation aimed at safety, sustainability, and utility.
Animal studies from the mid-twentieth century flagged this compound for moderate acute toxicity, with oral LD50s in the hundreds of milligrams per kilogram for rodents. Dermatological researchers have documented cases of contact dermatitis and, with repeated exposure, lasting skin and lung inflammation. Cell biologists link the compound’s cytotoxic effect to its ability to disrupt mitochondrial function and generate reactive oxygen species. Regulatory agencies push manufacturers to design out unnecessary exposures and handle all waste streams with controls that stop runoffs from reaching water tables. Over time, peer-reviewed studies built up an undeniable risk profile, prompting industry and regulators alike to keep tighter checks on who uses this compound, how, and where.
The ways forward all seem to hinge on balancing utility and safety. Demands keep growing in the specialty electronics and analytical reagent markets thanks to expanding biotech and renewable energy research. At the same time, the chemical community wrestles with tough questions about chronic low-dose exposure, environmental fate, and sustainable feedstocks. I can picture labs using automated handling robots and microfluidic reactors to minimize human contact in future production. Applied research aims at blending the compound’s redox power with greener sourcing—think electrochemical activation instead of gaseous chlorine, or even biotechnology routes using engineered bacteria to chlorinate natural quinones. The next decade will likely see greater transparency around manufacturing impacts, widespread digital tracking of reagent movement, and deep dives into biodegradable halogenated quinones. Responsible stewardship shapes the future for 2,5-Dichloro-1,4-Benzoquinone, or whatever name it picks up next.
2,5-Dichloro-1,4-benzoquinone doesn’t usually appear in headlines, but the role it plays carries real weight in both science labs and industries. Most people won’t find a bottle of this compound in their garage, yet its impact stretches from pharmaceuticals to environmental research. This chemical, often called DCBQ in technical circles, gives chemists a tool for getting deeper insights and building blocks that unlock other discoveries and products.
Often, the big story around chemicals like DCBQ is how they help make other chemicals possible. 2,5-Dichloro-1,4-benzoquinone acts as a strong oxidizing agent. In drug discovery, researchers use it to build new molecular structures. Without oxidizers like DCBQ, labs would hit roadblocks trying to build complex medicines, dyes, or electronic materials. It steps in to change molecules in ways that simpler chemicals just can’t handle.
For example, during the synthesis of antimalarial compounds and antibiotics, DCBQ can trigger key reactions that produce those life-saving molecules. I’ve spoken with chemists who keep this compound on their shelf because it gets unique results that other oxidants just don’t deliver. That matters when deadlines are tight and resources run thin.
DCBQ also acts as a test for detecting certain pesticides and herbicides in the environment. Research labs analyzing water or soil samples look for traces of pollutants, and DCBQ provides a way to bring those results to light. Scientists use the chemical in methods called colorimetric assays, where a visible color change signals the presence of contaminants. With tighter environmental rules, quick and reliable identification helps protect water and food.
A few years back, I watched a team running field tests at a polluted river site. The use of DCBQ turned complicated chemical measurements into something as clear as a color change in a test tube. Fast results meant a faster response for cleanup crews and public health teams. Data from groups like the U.S. Environmental Protection Agency underline how timely detection keeps contaminants out of drinking water and farms.
Of course, working with strong oxidizers calls for care. DCBQ can irritate the skin and lungs, so labs must follow strict safety steps. Disposal brings another side of responsibility. When handled poorly, DCBQ adds to the toxic load in landfills and streams. That’s why training, protective equipment, and proper waste management should remain front-and-center. Companies and universities now face more pressure to document safe chemical use — both for safety and for public trust.
More research groups are searching for safer or greener oxidizers. Alternatives can slash waste, reduce health risks, and even cut costs. Green chemistry is not just a slogan anymore. Still, chemicals like DCBQ stick around because they are deeply effective in certain settings, especially for tasks where nothing else works as well. So, smart use and a firm commitment to safety make the difference between progress and problems.
Progress depends on understanding both the uses and the costs of each tool we pick up. In the right hands, 2,5-dichloro-1,4-benzoquinone helps solve challenges faster, reveal what’s hidden, and deliver better outcomes—whether in a pharmaceutical lab or in the fight for cleaner rivers and food supplies.
Long hours in a lab or plant teach one lesson above all—chemicals don’t care who you are. 2,5-Dichloro-1,4-Benzoquinone sits among the more temperamental compounds. Touch it with bare skin and you may end your shift with burning or irritation that sticks around. Inhale a bit or let the vapors drift too close and you’ll understand why material safety data sheets seem so grim.
The risks aren’t just a short-term nuisance. Studies link some quinones to respiratory problems and possible long-term effects. Getting careless with storage or cleanup doesn’t simply leave a mess; it can harm everyone down the line—custodians, waste haulers, and even the next person who steps into the workspace.
Work with this compound in a fume hood. Don’t just crack a window; fumes can travel, and there’s no good reason to guess how irritating the air might get when there’s better protection an arm’s reach away. Ventilation keeps harsh chemicals out of your lungs and lets you skip that annoying cough that seems to show up after a day with poor airflow.
Nitrile gloves shield your skin from direct contact. Latex often won't cut it. If the quinone breaks through, swap them out. Double-gloving brings peace of mind, especially for longer projects—skin burns or rashes don’t fade quickly.
Splash goggles matter more than most realize. A careless splash to the eye sends many to urgent care each year—and it doesn’t matter if it’s your hundredth experiment or first week on the job. Don a lab coat or chemical-resistant apron to keep your clothes and arms clear.
Accidents still surprise even seasoned chemists. Keep a bottle of eyewash and an emergency shower nearby. Quick rinsing helps cut down long-term injury. If an incident happens, ditch contaminated clothes and shower off thoroughly—don’t just hope for the best.
Store the benzoquinone in a cool, dry space, away from common acids, bases, or reducing agents. These chemical mixes raise the odds of a runaway reaction. Keep the bottle clearly labeled and out of high-traffic paths, so no one stumbles or bumps into it on a busy day.
Handle waste right—pouring leftovers down the sink may sound tempting when you’re running late, but this practice endangers the water supply and likely breaks local regulations. Collect liquid waste in a sealed, properly marked container; let your designated disposal crew take over from there.
Glassware and surfaces demand extra scrubbing. A mild detergent and lots of rinse cycles remove stubborn residues. Skip shortcuts here; even trace contamination may spoil upcoming work or trigger health complaints among your colleagues.
Understand what you’re working with before reaching for a pipette. The chemical might seem like just one more reagent, but its hazards deserve real respect. Before tackling any project, review the latest guidance from OSHA, your institution’s safety officer, and up-to-date resources from trusted groups like the American Chemical Society.
Staying safe doesn’t mean living in fear or dodging chemicals altogether. It means knowing the risks and having reliable routines in place, so you can focus on results—instead of running damage control.
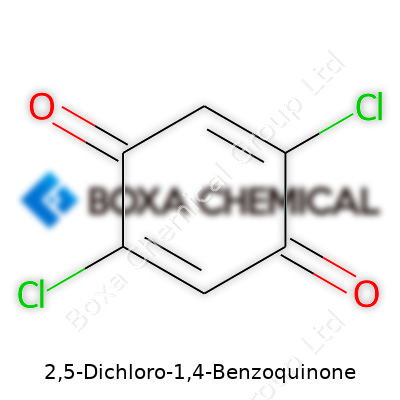
My first experience with 2,5-Dichloro-1,4-Benzoquinone didn’t start in a lab. It really came from trying not to cough every time I cracked open a bottle of chlorinated compounds while working as a student researcher. This molecule, with the formula C6H2Cl2O2, looks pretty simple on paper: a six-membered benzene ring, two oxygens at opposite corners (the 1 and 4 position, that’s what makes it a quinone), and two sharp, green-labeled chlorine atoms sitting at the 2 and 5 spots. I would sketch it on a page as a symmetrical ring, double bonds every other side, oxygens pointing off in a rigid square, and the chlorines sticking out across from each other. Nothing abstract or mysterious—just straight-up organic chemistry.
Some folks see a chemical formula and think it’s just for chemists. Looking closer, you see that swapping atoms on a ring can mean the difference between a helpful industrial tool and a toxic spill. In this case, the two chlorines on 1,4-benzoquinone jazz up the molecule’s reactivity. That’s part of why it finds use in syntheses or as an intermediate in making dyes, herbicides, and pharmaceuticals. Its straightforward, flat structure helps it squeeze into reactions where it acts as an oxidizing agent, handing off electrons easily.
I remember running thin-layer chromatography in grad school labs—with this molecule, even faint spots glared under the lamp, reminding you not to take it lightly. It packs a punch in chemical processes, and that reactivity ties straight back to where those chlorine atoms are parked on the ring.
Once, a colleague spilled a small amount in the hood and the whole workspace filled with a sharp, chemical tang. This experience hammered home the lesson that structure isn’t just about drawings in a textbook. The very atoms and bonds in 2,5-Dichloro-1,4-Benzoquinone help make it a potential health hazard—irritation, persistent fumes, and the possibility for worse if not handled right. Chlorinated benzoquinones linger in the environment, don’t break down easily, and can slip into waterways unless checked.
Science gives the facts; lived experience drives home the responsibility. Hazard communication and strict waste stream control make all the difference. A lab that treats this compound as just another yellow powder—without eye-wash stations, fume hoods, or proper disposal—ends up risking much more than a failed experiment.
Grasping the difference between 2,5-Dichloro-1,4-Benzoquinone and its many close cousins isn’t about ticking boxes on a safety assessment form. It’s about knowing what happens when an electron-loving molecule, loaded with chlorine atoms, finds its way onto a glove or into a reaction flask. That knowledge changes the way scientists plan, the questions industry experts raise, and the safeguards regulators put in place.
Respect for reactivity starts with training. Every chemist and worker around these substances benefits from seeing firsthand what a little spill or a stray whiff can mean. Good labeling, real-time air monitoring, and strict storage rules cut down on avoidable mishaps. Working with less hazardous alternatives whenever possible and following both local and international guidelines helps keep the balance between productivity and health. It’s easy to forget the importance of proper ventilation until the sharp smell hits you.
Anyone who has spent a little time working around chemicals knows some substances demand a bit more respect. 2,5-Dichloro-1,4-Benzoquinone doesn’t make headlines like some industrial material, but it comes with safety baggage that can’t be ignored. This compound is used in laboratories and for certain synthesis processes. It has a reputation for being an irritant to the eyes, lungs, and skin. Pinning down the right storage methods isn’t a luxury. It’s about protecting health, staying clear of environmental trouble, and avoiding wasted product. I once watched a colleague take a loose-capped container off a shelf. The sharp chemical odor hit hard, and we spent the next twenty minutes airing out the lab. Ever since, I’ve put extra care into how these quinones get stored.
Secure storage starts with a cool, dry, and well-ventilated spot. Humidity works against stability, often speeding up breakdown or causing clumping and contamination. Moisture in the air can slowly react with the compound, which leads to a mess and possible safety hazards. One lesson from years of hands-on work: keep containers tightly sealed. Screw-top amber glass jars provide a good barrier and block out light, which helps. Light exposure speeds up degradation and may alter how the compound behaves. Metal shelves sometimes react with spilled chemicals, so I go for powder-coated or plastic racks. Nothing beats a storage cabinet designed for organics, if the budget allows.
Labels turn out to be more than just a formality. Each jar or bottle should tell what’s inside, hazard information, and a legible date. I once found a mystery jar in a shared storage area that delayed an entire project day because nobody wanted to take a gamble. Experienced lab folks make it a habit to record the date of receipt and opening, tracking any color changes along the way. It’s a small act that pays off with peace of mind down the road.
Storing chemicals isn’t just about finding an empty shelf. Substances like 2,5-Dichloro-1,4-Benzoquinone do better alone. Keep oxidizers and flammable solvents at a good distance; accidental mixing can turn a quiet lab into a disaster scene. I’ve seen situations where poor organization meant incompatible chemicals landed next to each other, turning a routine inventory check into a stressful, sweaty afternoon. Separating these compounds by type cuts risk and helps keep things orderly.
Controlling who can grab these chemicals goes a long way. Staff should know what they’re dealing with, how to respond if something spills, and why following protocols matters. Safety data sheets belong right near the storage area for easy reference. Everyone working with this compound benefits from basic training and refreshers. A team that understands chemical behavior makes fewer mistakes and helps keep others safe.
Many accidents trace back to complacency or outdated routines. Audits and storage checks reveal old habits and help encourage better ones. Adopting standardized containers, digital inventory logs, and updated signage all make a difference. Beyond a personal sense of caution, institutions carry a responsibility to provide training and keep guidelines current. Modern storage solutions, like ventilated cabinets with built-in sensors for leaks or temperature changes, can tip the scales toward safety and efficiency.
Storing 2,5-Dichloro-1,4-Benzoquinone safely doesn’t require rocket science, but it does call for consistency, attention, and a respect for the risks involved. From tightly sealed bottles to organized storage spaces, thoughtful choices stand out as the best answer to a safer workplace. The habits learned early in the lab or plant stick around, shaping a culture where safety comes first and peace of mind follows.
Open a laboratory cabinet and spot a small glass jar with pale-yellow, needle-like crystals—chances are it could be 2,5-Dichloro-1,4-Benzoquinone. This solid doesn’t come with any pleasant aromas; a sharp, acrid smell hits hard instead. Such warning scents usually mean caution is needed, and that holds true here. The compound stays stable at room temperature, avoiding the drama of sudden reactivity. Toss it in water or let it sit in damp air, though, and you’ll slowly notice changes. It doesn’t dissolve well in water—just a trace manages to break through. Add it to organic solvents, like ether or chloroform, and it blends in a lot better.
Watch how this compound interacts with the world. 2,5-Dichloro-1,4-Benzoquinone melts at about 153°C, which is much higher than most familiar household chemicals. If you have spent hours over beakers and Bunsen burners, you recognize the importance of a stable melting point—consistency means safer handling and reliable reactions. Light or heat doesn’t bother it much, but strong bases or acids break it down, changing both its color and structure. In the chemical world, color changes aren’t just pretty—they warn of reactions you might not want to get out of hand.
Looking at its chemical structure, you see two chlorine atoms sitting on a benzoquinone ring. Both increase its toughness and make it less friendly. With this setup, 2,5-Dichloro-1,4-Benzoquinone acts as a mild oxidizer. That means people who work with it have to respect its reactivity, since it wants to snag electrons from other chemicals. In practice, that can cause headaches if it ends up in places it shouldn’t—nobody wants an unexpected oxidation ruining a batch experiment or corroding equipment.
Having chlorine atoms tacked on makes the molecule harder to break down. That presents both a blessing and a curse. In the lab, its durability keeps it from falling apart during reactions, so it serves as a useful intermediate in processes like dye or pharmaceutical manufacturing. Out in the environment, that persistence brings up concern. Water treatment specialists worry about byproducts like this in drinking water systems. Chlorinated quinones sometimes slip through old purification setups, posing health threats to local communities. Some studies, such as those published by the U.S. EPA and peer-reviewed toxicology reviews, highlight how repeated exposure at even low levels might disrupt normal cell function.
I remember working in a chemical warehouse years ago—handling compounds that could make or break someone’s day. Precautions with 2,5-Dichloro-1,4-Benzoquinone always included gloves, goggles, and strong ventilation; the safety data sheets didn’t sugarcoat its risks. Getting this stuff on your skin or breathing in dust isn’t harmless—short-term exposure might cause irritation, while long-term effects still raise unanswered questions among toxicologists.
Regulators look closer now at persistent chemicals like this. Upgrading municipal water filters with activated carbon or advanced oxidation holds potential. Researchers keep hunting for greener substitutes—molecules that do the same job without sticking around so stubbornly afterward. Manufacturers who handle 2,5-Dichloro-1,4-Benzoquinone in bulk bear the responsibility of proper containment and waste management; I’ve seen companies introduce onsite chlorine-neutralizing scrubbers, reducing the risk of accidents and off-site pollution.
Lessons learned from experience and research suggest a simple truth: a compound’s physical and chemical properties aren’t just textbook facts—they touch daily life, drive choices in industry, and shape decisions about public safety. If more folks understood what hides behind a label, safer solutions would keep gaining ground.
| Names | |
| Preferred IUPAC name | 2,5-dichlorocyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˌdaɪˌklɔːroʊˌbɛnzoʊkwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 615-92-7 |
| Beilstein Reference | 1203690 |
| ChEBI | CHEBI:47427 |
| ChEMBL | CHEMBL451184 |
| ChemSpider | 20424 |
| DrugBank | DB07742 |
| ECHA InfoCard | 03b8e338-8694-4ec6-8aaf-d2505795bc7e |
| EC Number | 204-399-4 |
| Gmelin Reference | 110062 |
| KEGG | C06466 |
| MeSH | D003856 |
| PubChem CID | 8514 |
| RTECS number | DJ9625000 |
| UNII | L1P7R8KP8X |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C6Cl2O2 |
| Molar mass | 157.00 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | chlorine-like |
| Density | 1.600 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.67 |
| Vapor pressure | 0.008 mmHg (25 °C) |
| Acidity (pKa) | -0.7 |
| Basicity (pKb) | 12.67 |
| Magnetic susceptibility (χ) | -47.6 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.613 |
| Viscosity | 1.39 mPa·s (25 °C) |
| Dipole moment | 2.60 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -108.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -471 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P210, P264, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P363, P403+P233, P501 |
| Flash point | 79 °C (closed cup) |
| Autoignition temperature | 165 °C |
| Lethal dose or concentration | LD50 Oral Rat 130 mg/kg |
| LD50 (median dose) | 170 mg/kg (Rat, oral) |
| NIOSH | DD9625000 |
| PEL (Permissible) | PEL: 0.1 mg/m3 |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | Unknown |