2,5-Di-Tert-Butylhydroquinone, known to many by its abbreviation DTBHQ, made its entry into the world of chemistry thanks to the relentless curiosity of researchers hustling through the 20th century’s deeper layers of antioxidant chemistry. The growing pursuit for chemically resilient antioxidants pushed scientists to experiment with hindered phenols. With its two whopping tert-butyl groups, DTBHQ offered exactly that: a hydroquinone core protected from unwanted oxidation, even under hostile conditions. That robust structure got the attention of industries dealing with fats and polymers—spaces that chewed through antioxidants quickly. Many in the older generation will remember the shift through the ‘70s and ‘80s, as food and plastics manufacturers tried to improve both stability and safety of their products. It’s in this period others saw the sense in using something bulkier, more stable, and less reactive, and DTBHQ’s adoption steadily grew.
From refinery floors to lab benches cluttered with vials, 2,5-Di-Tert-Butylhydroquinone has become a recognizable solid. Industries looking to extend shelf life or prevent the foul smell of rancidity in oils and polymers grab for DTBHQ. Its antioxidant punch serves not just the obvious market of food preservatives—synthetic rubbers and fuels have benefited, too. Chemists appreciate the compound because it delivers consistent antioxidative properties and resists decomposition in environments that wreck lesser molecules. It turns up in technical datasheets whisked around global labs, appearing as white crystalline solid in jars labeled with both care and authority.
Set a sample of DTBHQ on the scale and you’re looking at a solid that stands out with sheer persistence. Its melting point hovers from 138°C to 140°C—a bit of heat, but hardly enough to shake apart its sturdy skeleton. Slide waves of light through it, and you’ll find a UV-Vis signature typical for phenolic compounds; strong absorption in the UV region, a mark of antioxidant activity. Chemically, DTBHQ’s two tert-butyl groups at the 2 and 5 positions don’t just make it bulky—they build a wall that blocks unwanted reagents, preventing quick breakdown or polymerization. Those same bulky groups make the compound low in volatility and slow to dissolve in water, but an easy candidate for organic solvents like benzene, ether, or acetone.
A technical sheet for DTBHQ paints a clear picture. Purity remains a deciding factor for most buyers—99% or greater, often listed. Moisture content hovers near zero, reducing the risk of unwanted hydrolysis or oxidation in storage. Impurities, particularly other phenolic byproducts, rarely reach beyond 0.5%. Labels shout safety warnings in bold. The signal word “Warning” appears, followed by the compound’s proper name, synonyms, hazard pictograms, and advice on storage—cool, dry, isolated from strong oxidants. Each container wears an identity tag with the lot number, batch date, and often a scannable code for traceability. That attention to detail isn’t just formality—it’s the backbone of safe, consistent use, especially in sectors that fear recalls or accidents.
Producing DTBHQ calls for crisp technique. The most direct industrial synthesis begins with hydroquinone, closing in on it with tert-butyl alcohol and an acid catalyst—usually sulfuric acid. The reaction draws on strong acid conditions to drive alkylation onto the benzene ring. Careful temperature control keeps the tert-butyl groups on the 2 and 5 spots, avoiding junk products at the wrong positions. Once the mixture cools, solvents and repeated recrystallizations pull off the side products, leaving behind the measured, tidy crystals of DTBHQ. Labs in my own experience invest hours here for a handful of grams, just for testing; factories, on the other hand, churn out kilos daily, their pipes humming and distillation units burbling in lockstep.
DTBHQ loves its status as a stubborn molecule, but clever chemists squeeze utility from its structure. Under strong oxidizing environments, the hydroquinone moiety flips over to the corresponding quinone—much like its simpler cousin, benzoquinone. These reversible oxidation-reduction properties underpin DTBHQ’s value as an antioxidant, catching free radicals and handing out electrons to neutralize them. With enough heat and potent acid, the tert-butyl groups won’t always hang on; decomposition yields other phenols and some interesting hydrocarbon fragments, which have been profiled in the environmental monitoring world. Some niche research journals describe the creation of DTBHQ-linked polymers, grafted onto polymer chains, providing extended antioxidant action. The core structure takes well to etherification and acylation when given enough force, producing modified antioxidants for custom applications.
Depending on the catalog or the country of origin, one might bump into several names for DTBHQ. Some documents list it as 2,5-Bis(1,1-Dimethylethyl)-1,4-Benzenediol. Catalogs from Europe occasionally swap in EINECS identifier 207-414-8. Names like DTBHQ, Di-Tert-butylhydroquinone, and 2,5-Di-tert-butyl-p-hydroquinone turn up in procurement records, while older chemical supply houses might file it under less-used abbreviations, a relic from an earlier generation of paperwork.
DTBHQ’s safety sheet demands attention. Direct skin or eye contact leads to irritation, with workers reporting redness or rash after a spill. Finer powder proves a risk if inhaled, especially in tight factory spaces where ventilation isn’t up to par. Many chemists recall the acrid smell that arises when a bottle is left open too long; this isn’t just a nuisance, but a warning sign to store the product properly. Regulatory bodies like OSHA and ECHA expect operators to use gloves and goggles, to keep DTBHQ far away from ignition sources and oxidizers, and to have spill kits handy. Companies that take these steps see fewer chemical accidents and happier, healthier staff. Waste disposal follows local rules, but burning or dumping is out—the law expects collection and proper chemical destruction.
DTBHQ’s action as a stabilizer turns up across a swath of industries. The food sector made it famous as an additive that stretches the shelf life of edible oils and fats. Polymeric materials, especially those prone to sunlight and oxygen attack, benefit from a well-blended pinch of DTBHQ. Lubricants, fuel blends, and synthetic rubbers appreciate its bulwark against peroxidation and color change. Producers of adhesive resins and varnishes include it for the same reasons. Research labs continue to find new territory—in specialty coatings, detergents, and even pharmaceuticals that need an extra wall between delicate active ingredients and air.
Research around DTBHQ continues to dig deeper into new application spaces and mechanisms of action. Teams push for improved process safety and reactor throughput, squeezing down costs and environmental impacts of large-scale production. Some look into grafting DTBHQ onto polymer backbones, creating smarter, longer-lasting antioxidant materials. Methods for greener synthesis—swapping out strong acid catalysts for solid-state options or pursuing solvent-free reaction schemes—show up regularly in recent journals. Others explore environmental fate and pathways for breakdown, seeking comfort in biodegradation profiles and eco-toxicity benchmarks. Academic groups push the limits of DTBHQ in electronics and sustainable materials, blending chemical tradition with urge for a greener, tech-driven world.
Toxicity studies around DTBHQ reveal a layered picture. Acute oral toxicity falls in the moderate range for rodents, and chronic exposure causes mild liver changes at doses above occupational limits. Companies who run animal studies find skin and eye irritation at doses relevant to normal use, but no clear cancer risk in long-term tests. Regulators push for insight about what happens after DTBHQ breaks down in soil or water, especially as trace residues turn up in waste streams. Human workplace studies flag the potential for contact dermatitis in susceptible employees. For now, though, the overall risk remains far less than older, dirtier phenolic antioxidants that it sometimes replaces.
The horizon for DTBHQ shows steady momentum as industries continue hunting for high-performance antioxidants that avoid toxic baggage. With bans and regulatory attention raining down on older phenolic additives, DTBHQ’s blend of stability and moderate toxicity keeps it in strong demand. The market could pivot on research breakthroughs—such as bio-based synthesis or recyclable composites—linking chemical know-how with modern sustainability goals. Fields like battery research, advanced coatings, and drug formulation may yet tap deeper benefits from this sturdy molecule. As work continues on alternatives, DTBHQ stands as both a benchmark and a springboard, a compound tested by decades of industry and research, and still with more to offer those who look beyond the usual formulas.
2,5-Di-Tert-Butylhydroquinone, more often known as DTBHQ, shows up in labs, factories, and sometimes in the margins of food production. This chemical acts as an antioxidant, which means it keeps things from going bad too fast. Consider vegetable oils, which spoil and turn rancid because of oxygen. DTBHQ steps in and slows that process, keeping oil fresh for longer.
Back in my days working in a food research lab, we ran shelf-life tests on a lot of snacks. Sometimes, oils would smell off long before the packaging actually broke down. Incorporating antioxidants changed everything—DTBHQ stood out for its reliability under high-temperature storage. These antioxidants don’t just hold back spoilage; they help companies avoid product recalls, bad odors, and customer complaints.
A less obvious but just as important use comes from plastics manufacturing. DTBHQ finds a home in the production of polymers and rubber, where it stops chemical reactions from running wild. Uncontrolled polymerization can cause dangerous pressure build-up, ruined product, and sometimes injured workers. Chemical plants rely on DTBHQ as a stabilizer, making their production both safer and more consistent.
Back in college, a visit to a local packaging plant left me with strong memories—one wall lined with safety posters showing reaction vessels and the risks of runaway reactions. Those places depend on stabilizers like DTBHQ, and it’s easy to see why.
DTBHQ gets attention from regulators who want to make sure food is safe. Studies have examined whether DTBHQ poses health risks. The FDA and similar agencies around the world have set limits on how much can be added to food, always looking for a balance between safety and keeping food fresh.
Experience shows that most consumers don’t even notice this ingredient in their foods. The real test comes down to regulation and science. Research reviews track both the benefit of keeping food edible and possible toxic effects. In my reading, I found that most people eat far less than the allowed limits, especially since DTBHQ stays in very specific products and amounts.
No chemical addition comes without some impact. Waste streams from food and plastics manufacturing, for example, sometimes contain traces of antioxidants. I used to work on a university study that looked at how these compounds show up in water samples downstream of plants. While amounts were low, tracking and filtering these byproducts can make a difference in ecosystem health.
Better waste treatment methods, like activated carbon (which traps DTBHQ and similar molecules), can cut down the levels that escape. Manufacturers who take the initiative to invest in efficient clean-up contribute to both environmental protection and ongoing use of helpful additives.
DTBHQ supports industries from packaged snacks to plastics. But the most successful businesses pay attention to both performance and public trust. That means using antioxidants only as much as necessary, staying up to date on scientific findings, and keeping communication open with both regulators and customers. Relying on new testing and smarter waste management gives everyone the best shot at safe food, clean water, and strong materials.
This antioxidant, known to many as DTBHQ, plays a small but important role in preserving fats and oils. You’ll spot it on some ingredient lists, though chemists often work with it in its pure form, which poses more risk than the bits found in food. Handling DTBHQ in a lab means treating it with the same respect anyone gives to unfamiliar powders—the margin for error shrinks drastically as exposure risks rise, especially in long work sessions.
Some chemists recall early days in undergrad labs, where the teacher harped on using gloves and fume hoods for everything. Experience quickly proves those rules aren’t just red tape. DTBHQ dust triggers respiratory discomfort, much like the time I cleaned a particularly grimy attic, only with more risk for lasting irritation. A spilled bottle easily turns breathable with routine tasks. That kind of exposure adds up, and the risk extends beyond immediate burning throats—benzene-based quinones can cause headaches, dermatitis, and longer-term problems if workers shrug off the warnings.
Skin contact leaves most people with red, irritated patches, and eye exposure delivers a sharp, burning sensation. Cheaply made goggles or torn gloves don’t cut it; a sudden itch near the eye can become a trip to the nurse. Clean labs use double-nitrile gloves, not latex, since chemicals like DTBHQ find routes through pinholes and thinner material. Anyone in the habit of removing safety glasses “just for a second” risks regretting it immediately.
DTBHQ’s fine crystalline powder travels in air currents, so a quality fume hood marks the safest space for weighing and transferring. Dust masks do their job, but a certified respirator builds an extra layer of confidence for those working with open beakers or scoops. Oversized chemical aprons keep sleeves from picking up invisible traces, and scrupulous handwashing keeps residues out of the break room and off the keyboard.
Chemistry teachers enforce spill kits near every workspace for a reason. Fast action—scooping up powder with spatulas and soaking rags in vinegar—makes a mess less likely to stick. Proper labeling keeps students from mistaking it for harmless sugar. Labeling isn’t just bureaucracy; it means fewer mistakes during cleanup, storage, or emergency aid.
One colleague once skipped routine allergen testing for a new hire, and a rash within minutes forced an early end to promising lab work. Allergic reactions can pop up out of nowhere with quinones. Wearing the proper gear makes a difference, but so does having eyewash stations and safety showers within arm’s reach. We always check expiration dates on the safety equipment itself; that expired bottle of saline could offer cold comfort in the wrong moment.
Training keeps risks from turning into accidents. Short, practical drills—focusing on what to do during exposure—stick better than handing out rulebooks. Facilities can rotate tasks to limit one person’s contact time, and regular air monitoring picks up what sight or smell can’t detect. Anyone running a lab or a food processing line can push for regular audits, better PPE, and ongoing feedback from ground-level staff, not just upper management. Greater transparency and continuous updates save skin, lungs, and time.
Anyone working with chemicals knows a lapse in storage can spark problems swiftly. In the case of 2,5-Di-Tert-Butylhydroquinone—a powerful antioxidant commonly used in food preservation and chemical manufacturing—the stakes climb higher. Keeping its potency and ensuring safety relies on remembering not just why storage matters, but what real-world risks lurk in everyday lab environments.
Keeping moisture out isn’t busywork—it’s protection. I’ve seen the headaches that sloppy cap tightening or damaged desiccators deliver: clumping, yellowing, and even hazardous reactions. This compound, like most hydroquinones, absorbs water from humid air and can start to degrade. A sealed container, with a well-fitted lid (think screw-cap over flimsy snap-tops), inside a desiccator turns that risk way down. You can add silica gel packs, but those desiccators often do the heavy lifting.
Bright rooms, direct sunlight, or heat near a vent all spell trouble. Ultraviolet rays spark chemical changes that break down antioxidants. I use amber glass bottles and pick a cabinet far from sunny windows or radiators. Refrigerators help, but only as long as frost and moisture stay out—frozen lumps won’t weigh or dissolve evenly in prep work. Storing at 2–8°C, or simple room temperature under 25°C, is often enough if both dryness and darkness team up.
Cross-contamination sneaks in through shared spatulas or open exposure. I always use separate, labeled scoops and return leftover powder to waste—not the main bottle. Glove changes and clean benches aren’t just lab protocol; they cut back on accidental adulteration that could throw off results or spoil the product. Take it from the countless botched reactions on record: fast and loose routines cost more time than strict habits ever do.
Minor spills seem harmless, but 2,5-Di-Tert-Butylhydroquinone can irritate the skin or airways. Handled carelessly, powders float up unseen. Working in a well-ventilated area or a fume hood, and using gloves, always keeps risks minimal. A spill kit with absorbent pads and a dedicated disposal bin right near storage cuts panic if something tips.
Nothing beats a clear, bold label—the date opened, and any odd changes in color help track shelf life. Old stock should move out before new, and I make a habit of monthly checks. It’s not just about avoiding waste; it heads off using degraded, less effective material.
Whether working solo or in a shared space, everyone’s safety depends on joint vigilance. Corporate setups often mandate Standard Operating Procedures because routines prevent disaster, especially with high-value or hazardous chemicals. Small lapses invite big cost, not just in lost product but in health and research credibility.
Those who take the time to store 2,5-Di-Tert-Butylhydroquinone well aren’t just ticking off a regulation box. Every sealed bottle, every checked temperature, and every clean return reflects real commitment to good science, workplace safety, and personal integrity. That’s a reputation worth protecting.
Many food labels carry long, complicated names that most of us ignore. 2,5-Di-Tert-Butylhydroquinone (DTBHQ) fits into this category. It's a synthetic antioxidant often used to preserve fats and oils, keeping food stable on the shelf. The food industry leans on chemicals like this to slow rancidity. If you check packages of snacks, instant noodles, or even cosmetics, you may find this compound lurking on the ingredient list.
Eating processed food exposes us to more additives than any other generation. Some, including DTBHQ, haven't been deeply studied. What is known points to caution. Lab studies show high DTBHQ doses can irritate skin, eyes, and the respiratory tract. Ingesting a lot of it led to stomach pain, vomiting, and even symptoms like tremors in animal studies. That said, most food regulations set allowable levels much lower than those used in toxicity experiments.
Long-term risks clouds the conversation more. Only limited studies probe what years of low-level DTBHQ exposure does. Some animal tests found DNA damage and altered immune response, which makes the risk hard to dismiss. No broad, human-based research can clear up the picture, and doctors don't see clear links between everyday consumption and serious health effects — at least not yet. Still, the potential for oxidative stress means those with weakened immune systems or allergies may face higher risks.
The journey doesn't end after a chip bag hits the landfill. DTBHQ ends up in sewage and runoff, meaning it can show up in rivers and soil. Aquatic organisms take the biggest hit. Studies find that even moderate concentrations disrupt fish reproductive cycles. Breakdown in the environment runs slow, which means risk builds over time rather than washing away after every rain.
Soil bacteria and beneficial insects don't escape trouble. Like most synthetic antioxidants, DTBHQ can accumulate in waste streams, subtly tweaking microbial life. This shift affects the whole local ecosystem, sometimes in ways researchers only discover after years of widespread use. Communities living near factories or treatment plants see more of these chemicals than anyone else — a classic case of environmental justice getting overlooked in pursuit of longer shelf life.
There's a strong case for keeping a closer eye on synthetic antioxidants. Food manufacturers could dial back their use, switching to natural options like rosemary extract or vitamin E. These work as well for slowing spoilage but have longer histories of food safety. Stronger labeling rules would help consumers make smarter choices, similar to what happened with trans fats in the past decade.
Governments should fund long-term studies on exposure, especially among high-risk populations. Environmental agencies need stricter monitoring for places downstream from processing plants. Food safety doesn't have to collide with environmental stewardship. As more research surfaces, steering away from the least-studied additives can bring big health and environmental rewards — a reminder that simple changes can go a long way.
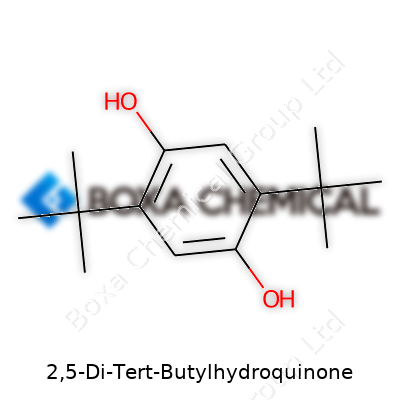
2,5-Di-Tert-Butylhydroquinone stands out in the world of chemical antioxidants because it takes the basic hydroquinone scaffold and adds two bulky tert-butyl groups at the 2 and 5 positions on the aromatic ring. Anyone who has spent time in a college lab or poured over chemical databases recognizes the classic shape: a benzene ring with two –OH groups lodging themselves at the para positions (these are on the 1 and 4 spots), but the real distinction comes from those tert-butyl additions. The tert-butyl group itself is pretty chunky—three methyl groups clustered on a central carbon—so plunking two of them on the ring both shields and stabilizes the hydroquinone core.
The molecular formula sums this all up as C14H22O2. It's easy to break it down: 14 carbons, 22 hydrogens, two oxygens. The visualization helps in grasping how the molecule reacts compared to regular hydroquinone—the extra tert-butyls add bulk and electron-pushing power, nudging the molecule toward better performance in certain industrial or laboratory settings.
The molecular weight pops right out of basic arithmetic: Carbons give 12.01 g/mol each, hydrogens add 1.01 g/mol, and each oxygen has 16.00 g/mol weight. All summed up, the total rounds to 222.32 g/mol. This matters for anyone looking to blend precise quantities in research, food, or even cosmetic preservative applications. You grab a scale, weigh out 222.32 grams, and you know you have a mole of pure 2,5-Di-Tert-Butylhydroquinone in hand. Such clarity makes formulation and quality checks manageable—not the headache it can be with unclear data.
Having a good handle on chemical makeup tells a bigger story. Those tert-butyl groups act as guards, protecting the core hydroquinone segment from quickly breaking down. In practice, this translates to better oxidative stability. If you've ever worked on keeping oils or plastics from degradation, these modifications show up in longer shelf life and improved resistance to temperature swings or exposure to air. It’s not just about making something last longer but also about protecting end users from potential byproducts that can arise when antioxidants fail to do their job.
The relatively high molecular weight, compared to regular hydroquinone (which only weighs 110.11 g/mol), cuts down on volatility. In environments where low molecular weight additives evaporate off—left unchecked, these losses can cause food or chemical formulations to fall apart—2,5-Di-Tert-Butylhydroquinone proves more reliable. This knock-on effect benefits industries aiming to lower waste or avoid sudden changes in product performance.
Safety plays a huge part in the ongoing debate about food and cosmetic additives. The structure of 2,5-Di-Tert-Butylhydroquinone can both help and complicate things. Those tert-butyl groups give stability, but researchers continue to investigate if any breakdown products create unwanted effects in humans or ecosystems. Real trust builds when studies back up claims, not just from industry but also from independent labs and regulatory agencies.
Pushing for transparency helps: clear labeling, open research, and robust monitoring of both benefits and risks. Showing molecular details and pathways, not hiding behind buzzwords, empowers both scientists and the public. And when updates come, they come from learning together—not from hiding behind technical fog. That’s how innovations in chemical science reach everyone safely and fairly.
| Names | |
| Preferred IUPAC name | 2-(2-Methylpropan-2-yl)-5-(2-methylpropan-2-yl)benzene-1,4-diol |
| Other names |
2,5-Di-tert-butylbenzene-1,4-diol 2,5-Di-tert-butyl-1,4-benzenediol 2,5-Di-tert-butylquinol DTBHQ |
| Pronunciation | /ˈtuː,faɪ dɪ tɜːrt ˌbɜːr.təl haɪdroʊkwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 88-58-4 |
| Beilstein Reference | 1635891 |
| ChEBI | CHEBI:34712 |
| ChEMBL | CHEMBL133360 |
| ChemSpider | 16001 |
| DrugBank | DB14015 |
| ECHA InfoCard | 03b754e4-8a4c-46c1-8101-04172ea4afee |
| EC Number | 201-793-8 |
| Gmelin Reference | 90650 |
| KEGG | C15997 |
| MeSH | D017849 |
| PubChem CID | 7409 |
| RTECS number | SX8575000 |
| UNII | YU9U09GAI4 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9014374 |
| Properties | |
| Chemical formula | C14H22O2 |
| Molar mass | 222.32 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 0.976 g/cm³ |
| Solubility in water | insoluble |
| log P | 3.53 |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | pKb = 10.04 |
| Magnetic susceptibility (χ) | -36.9·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.049 |
| Viscosity | 160 cP (25°C) |
| Dipole moment | 2.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -600.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –7168.9 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A16AX24 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0-Health-3-Flammability-0-Instability- |
| Flash point | 150 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1220 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 3200 mg/kg |
| NIOSH | DJ8925000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | Not established |