The journey of 2,4-Dichlorophenol traces back to the early twentieth century, a time when organic chemistry started fueling industrial growth. Laboratories in Europe and the United States first documented the chemical as researchers worked to synthesize phenolic compounds with antimicrobial potential. Factories did not just look for new molecules; they drove experimentation that provided cost-effective processes for large-scale synthesis. Over decades, increased interest in crop protection, pest control, and chemical intermediates gave 2,4-Dichlorophenol a new place in industry. I have seen how industries such as agriculture, pharmaceuticals, and plastics incorporated this compound, showing how scientific curiosity evolves into practical solutions that touch daily life.
2,4-Dichlorophenol stands out as a versatile chemical intermediate. Factories use this pale, crystalline solid to produce herbicides like 2,4-D, preservatives, and certain dyes. Its influence stretches far beyond the laboratory bench because its chemistry allows easy integration with other molecules, opening doors for countless end products. I have personally come across the unique, sharp odor of this compound while working in a safety-controlled environment, a reminder of its constant presence in manufacturing. Supply chains worldwide rely on timely production and safe transport, reflecting how one molecule shapes broad industrial ecosystems.
2,4-Dichlorophenol appears as off-white to light tan crystals, melting at about 41°C and boiling at around 210°C. Its solubility in water remains low—chemical engineers often turn to organic solvents like ethanol or ether for processing. The density averages 1.38 g/cm³, which influences how it settles and stores in tanks. With two chlorine atoms attached to the benzene ring, the molecule resists rapid breakdown under many conditions. Reactivity patterns show moderate activity: it handles chlorination, nitration, and coupling with other aromatic compounds, which offers pathways for further modification.
Industry standards demand clear technical parameters for 2,4-Dichlorophenol purities, usually set above 99%. Labels warn of potential hazards, including the risk of skin and eye irritation. UN numbers and hazard codes guide safe transportation, ensuring compliance with local and international laws. Material Safety Data Sheets list everything from safe storage—cool, dry, well-ventilated spaces—to required personal protective equipment, showing regulatory rigor born from decades of occupational health lessons.
Most production facilities make 2,4-Dichlorophenol by chlorinating phenol using chlorine gas in the presence of a catalyst, often iron(III) chloride. This process offers efficiency and cost control, both crucial for global markets. Plants recycle by-products and monitor emissions to stay aligned with environmental rules. Modern plants invest in closed systems and advanced filtration, steps driven not only by compliance—my experience in chemical safety shows this approach also helps safeguard workers and communities living near factories.
Chemists prize 2,4-Dichlorophenol for its ability to act as both a reactant and a modifier. Chlorination, nitration, and alkylation routes deliver precursors for growth in herbicide, dye, and antimicrobial sectors. In the lab, electrophilic substitution reactions allow tailored synthesis—every small adjustment to the chemical structure leads to a possible new function. I have watched process engineers optimize conditions, lowering costs and emissions while improving yields—a clear case for how innovation can support both productivity and environmental stewardship.
Depending on region and marketing, 2,4-Dichlorophenol appears under names like 1-Hydroxy-2,4-dichlorobenzene, Benzene, 1,3-dichloro-4-hydroxy, or its simple abbreviation 2,4-DCP. Catalogues in global supply houses list all these names to help clients source the right material and ensure regulatory clarity. The variety of names can lead to confusion, particularly for those less familiar with chemical nomenclature, so suppliers and users depend on standardized identifiers such as CAS numbers.
Strict occupational standards surround the use of 2,4-Dichlorophenol. Gloves, goggles, and fume hoods provide frontline defense against accidental exposure. Regular monitoring tracks airborne levels and surface contamination. Regulations worldwide specify exposure limits, with agencies like OSHA and the European Chemicals Agency leading oversight. Despite advances, accidents can still happen; that underscores the importance of ongoing training, emergency preparedness, and investment in new technology for containment and air filtering. Facility audits and continual education drive safety records forward.
Manufacturers turn 2,4-Dichlorophenol into products beyond simple bulk chemicals. It serves as a keystone intermediate for synthesizing herbicides, especially those controlling broad-leaf weeds in cereal crops. The paint and textile sectors rely on it for dyes and pigment precursors. Disinfectant production also draws heavily on its unique chemistry. In water treatment, modified forms help control bacterial and algal growth. These practical uses shape industries that support food security, public health, and infrastructure, all from the backbone of chemical process innovation.
Scientific research circles around both the risks and untapped potential of 2,4-Dichlorophenol. Newer synthetic paths focus on greener chemistry, lowering by-product loads and energy use. Analytical chemists study breakdown pathways to design better waste treatment. Teams look for catalysts that boost yield without hazardous residues. Ongoing research investigates novel herbicidal and antimicrobial candidates derived from 2,4-Dichlorophenol. My experience leads me to believe that research budgets, collaboration with universities, and partnerships with environmental teams enable safer, more effective innovations each year.
Toxicologists have collected years of data on the impact of 2,4-Dichlorophenol on human health and the environment. Even small spills can harm aquatic systems, where this compound disturbs reproduction and growth in fish and amphibians. Acute exposure in humans can lead to irritation, headaches, and, at high doses, more serious effects. Chronic exposures carry greater risk, pushing for ever-lower workplace exposure limits. My past visits to remediation sites established that contaminated soils and water frequently result from legacy mismanagement, making clean-up long and expensive. This history underlines the need for stronger controls, real-time monitoring, and public transparency about chemical risks.
As sustainability gains ground, industries rethink reliance on chlorinated aromatics. Circular chemistry, more robust remediation tech, and biological alternatives guide future directions. Regulatory bodies respond by tightening controls and encouraging investment in non-chlorinated pathways. Startups experiment with biological catalysts that break down phenolic compounds with less mess and more energy efficiency. In my conversations with chemists and plant managers, there’s hope that ongoing investment will deliver safer, cheaper alternatives without sacrificing the benefits modern chemistry brings to agriculture, healthcare, and materials science. The future of 2,4-Dichlorophenol, like so many compounds discovered in the early days of synthesis, hinges on how society balances utility, risk, and responsibility.
2,4-Dichlorophenol doesn’t show up in news headlines quite like some other chemicals, but its presence underpins a host of everyday processes. As someone who’s spent years digging through both scientific research and practical stories from farmers and manufacturers, I’ve seen that this compound holds a place of quiet significance in both agriculture and industry.
The main job for 2,4-Dichlorophenol starts in the production of herbicides. It’s a crucial building block for making 2,4-D, an herbicide that’s been in steady use since the 1940s. Across the world, farmers rely on 2,4-D to keep broadleaf weeds from choking out their crops like corn and wheat. Without these selective weed killers, yields would fall and food prices would climb. Crop scientists attest that using 2,4-D based solutions allows growers to focus more on crop health and less on back-breaking mechanical weeding.
Besides agriculture, 2,4-Dichlorophenol acts as a starting point for many other synthetic chemicals. Companies use it to make antiseptics, dyes, and even some pharmaceuticals. A lot of the preservatives that stop goods from spoiling too quickly in storage owe their shelf life to chemicals that start with this molecule. In factories, precision matters, and the reliability of 2,4-Dichlorophenol as a building block keeps production lines rolling efficiently.
There's a flip side, and it’s something worth considering. Groups like the Environmental Protection Agency and the World Health Organization have careful eyes on substances made from or containing 2,4-Dichlorophenol. Improper handling, poor disposal, or accidental leaks at the factory stage can put communities at risk. Runoff from pesticide-treated fields sometimes brings trace amounts into streams and lakes, disrupting aquatic ecosystems or even ending up in drinking water. I’ve spoken with environmental watchdogs who push for better reporting and more modern treatment facilities at both ends of the production chain.
Some manufacturers and farmers have taken proactive steps by adopting stricter controls over how these chemicals are made and used. The rise of integrated pest management, which combines herbicides with natural weed-control measures, points to smarter use rather than more use. There’s movement in research labs as well; scientists are experimenting with alternative weed-control compounds that break down more quickly in the environment.
The demand for transparency has never been clearer. In my own work tracing the lifecycle of industrial chemicals, the recurring truth is that informed companies, governments, and consumers can drive higher standards. Clear labeling, better worker training, and honest risk reporting matter just as much as the chemical itself.
2,4-Dichlorophenol finds its way into a surprising range of products and fields. Its story isn’t just about industrial power—it’s about the challenge of balancing productivity with stewardship, and the ability of science and policy to find safer paths forward. The future probably won’t erase chemicals like this any time soon, but there’s every reason for those who use and regulate them to keep raising the bar for safety and responsibility.
2,4-Dichlorophenol shapes a lot of discussions in industry labs. This compound doesn’t just show up in dusty jars, but appears in all sorts of places—chemical synthesis, pesticide work, even in some plastics manufacturing. But it packs quite a punch when handled wrong. This is not the kind of stuff you want on your skin or splashing near your eyes.
No one shakes off a chemical burn, and I learned early in my lab days that nitrile gloves, a solid lab coat, and closed shoes offer more than just comfort. Long sleeves protect arms from stray droplets. Goggles stay on, since splashes can land before anyone blinks. The fumes aren’t welcome in lungs either, so a well-fitted respirator or cartridge mask matters, especially during transfers or mixing. Open windows won’t cut it. Work gets done under a fume hood with ducts that actively pull vapors out.
Storage happens before spills even enter the picture. 2,4-Dichlorophenol belongs in robust containers with lids that won’t pop off. Storing it low on sturdy shelves keeps it out of reach if a container falls. Every container gets a big, bold label—there’s no excuse for guesswork here. Fire risk lurks because this stuff catches fire more easily than people think, so no storing near flames, heaters, or direct sunlight.
A good spill kit sits nearby. Kits need absorbent pads, neutralizing agents, and plenty of gloves. Spilled material shouldn’t dry out on surfaces. If a spill happens, people leave the splash zone and only trained workers pick up the mess. No quick fixes—mops just spread the chemical around. People don’t touch their phones or personal items until hands get washed thoroughly.
Emergency plans bring peace of mind and quick action. Every worker knows: Eyes? Rinse for at least fifteen minutes. Skin? Wash off with soap, then forget pride and find medical help. Fumes or ingestion? Keep the chemical’s label ready for doctors, since treatment often depends on knowing the precise exposure. Having a printout of a safety data sheet in an easy-to-reach spot shortens response times.
An untrained hand can endanger many. Refresher training shows how easy it can be to let habits slip. Team leaders build repeating schedules, drill on clean-up technique, understand cross-contamination, and keep logs updated. Teaching goes both ways. Workers give feedback, raising concerns about shifting storage, aging containers, or new procedures. This kind of open discussion keeps everyone on their toes.
Disposing of 2,4-Dichlorophenol takes more than dropping it in a bin. Certified disposal companies know what to do with it. Proper documentation follows every container, and nothing leaves a facility unless it checks off every regulation. Unsafe dumping doesn’t just break rules—it wrecks water systems, wildlife, and reputations.
Lab culture depends on treating chemicals with healthy respect, not just following rote instructions. Building good habits, keeping gear handy, and practicing response plans shape a safer environment for everyone who walks through the door. Taking shortcuts or gambling with safety leaves damage that goes beyond just the workplace.
2,4-Dichlorophenol, sometimes found in herbicides or as a breakdown product of pesticides and disinfectants, can quietly work its way into soil, water, and even the air in places near manufacturing plants or farms. Most folks have never seen it, but it still manages to circle through our environment — and it’s not something people want in their backyard. Research and my own work in environmental studies have shown that these kinds of compounds rarely hang around without trouble.
Living near a waste site, working on farms, or drinking contaminated water opens the door to 2,4-Dichlorophenol exposure. Skin contact and breathing in vapor also let this chemical into the body. Years back, I worked on a team testing private wells in rural neighborhoods, and we were shocked to find traces of related chemicals in water folks used daily. The families had no idea.
The big concern with 2,4-Dichlorophenol comes from how it acts inside the body. Touching or inhaling this compound can make the skin itch and eyes water. If someone gets a strong dose — let’s say through a spill at work or by drinking polluted water — it could irritate the lungs and cause throat pain, coughing, or worse. I’ve talked with workers who developed rashes on their hands after just one shift mixing pesticide sprays.
The longer-term picture gets more worrying. Studies on lab animals have shown that high doses hurt the liver and kidneys over time. Endocrine disruption, meaning the body’s hormone systems get thrown out of whack, is another risk scientists continue to track. In 2022, a review in Environmental Health Perspectives found that similar chlorinated phenols could mess with thyroid function, possibly impacting growth and metabolism in children.
Some researchers suggest a link between regular chemical exposure and certain types of cancer, such as non-Hodgkin lymphoma. The data isn’t carved in stone for 2,4-Dichlorophenol specifically, but the connections prompt people to ask tough questions. Nobody enjoys living with that kind of uncertainty.
Prevention makes sense. Filtering drinking water with activated carbon stands out as an effective way to catch unwanted chemicals like 2,4-Dichlorophenol. Hand protection and masks for workers who handle herbicides can really cut risk. In my own home, I use a certified filter because groundwater in my region sits close to fields where pesticides get sprayed.
Regulators and local watchdog groups also play a part. Testing water regularly and pushing for cleanups at known contamination sites help everyone sleep easier at night. Communities can benefit from public records that reveal which neighborhoods face the greatest exposure — a lesson learned after too many tragedies in polluted towns.
Choosing safer alternatives in yard care, supporting organic farming, and pushing for stronger product labels all matter. Every small step counts when it comes to keeping chemicals out of our lives and backyards.
Most folks don’t get up close to chemicals like 2,4-Dichlorophenol during their daily routines. I’ve walked through industrial and agricultural sites where products like this play a role, and it’s clear—even a small slip can lead to tightly sealed rooms full of headaches. This compound serves a range of uses, from making herbicides to popping up as a byproduct in wastewater. What worries me most is how careless storage or careless disposal can hit the soil, water, and air—just about every part of the environment that keeps communities healthy.
Anyone who’s been inside a chemical storeroom knows how quickly messes can escalate. 2,4-Dichlorophenol isn’t something you just dump onto a shelf. Poor storage can mean leaky drums or splashes on gloves, which can irritate skin and eyes, and breathing in the fumes often leaves folks coughing—sometimes worse. Once it leaches into groundwater, even tiny amounts can disrupt ecosystems and linger for years. There’s a reason regulatory bodies like the EPA and OSHA publish strict rules for handling it.
Every experienced facility manager I’ve met stresses the need for the right containers. Stainless steel or high-grade plastics with sturdy seals hold up best. Drums should sit off the floor on pallets, away from drains and sunlight. Chemicals love to mix in unpredictable ways, so I always recommend separating 2,4-Dichlorophenol from oxidizers, acids, and anything flammable. Ventilated rooms cut down on buildup of fumes. Labels should be legible—no smudged ink or fading. Emergency gear, like eyewash stations and spill kits, cannot just collect dust in the corner, since accidents happen to even the best teams.
I’ve seen mistakes up close—pouring chemicals into drains or tossing leftovers in the trash. That’s how you get sick workers and polluted waterways. 2,4-Dichlorophenol needs real chemical treatment, not just a trip to the landfill. High-temperature incinerators, designed for hazardous waste, break it down properly. Accredited hazardous waste contractors exist in every region, and they keep the chain of custody clean so nothing slips into the wrong hands. Local regulations often require manifest tracking, which helps communities hold facilities accountable. A chemical spill at a single warehouse can mean remediation and bad headlines for years.
Facilities do well to train workers on handling hazardous compounds from day one. In my years around industrial safety, repeated drills and clear operating manuals have saved more than a few people from close calls. Record-keeping—sometimes brushed off—is another key step, since lost paperwork makes it impossible to track old stock before it degrades. I’ve joined local community meetings where residents demand better transparency from companies that use chemicals like this, and those demands make an real impact.
Modern technology is catching up, and safer substitutes for 2,4-Dichlorophenol get more attention. But as long as this compound is around, the lessons are the same: stay vigilant, follow proven procedures, and make sure disposal fits both the law and common sense. Public health, worker safety, and the land beneath our feet depend on it every single day.
2,4-Dichlorophenol sits on the list of industrial chemicals used for everything from making herbicides to making antiseptics. Factories favor it for its role in chemical synthesis. The name shows up in the paperwork for pesticide ingredients, and it often traces back to the manufacture or breakdown of products you don't even notice, like certain wood preservatives and dyes.
The Environmental Protection Agency does keep tabs on 2,4-dichlorophenol in drinking water and surface water. It’s listed among hazardous substances under the Clean Water Act. The Occupational Safety and Health Administration sets strict exposure limits for workers in places where the chemical could linger in the air. In Europe, strict rules limit it under REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals). Canada includes this substance in its list of toxic chemicals. For all the scrutiny, gaps still exist. For example, in the U.S., the EPA only gives guidance for safe exposure in water. There’s no hard federal cap for its presence in finished drinking water, just a benchmark.
Rain washes 2,4-dichlorophenol off farm fields or out of waste dumps and into streams. It does not break down in a hurry, so it sticks around in water and soil. Fish and wildlife pick it up through contaminated environments. It can move up the food chain, from tiny bugs in the mud to the fish in your dinner. In a real-world sense, levels often rise around industrial hubs, and wastewater treatment plants sometimes pass it untreated.
Scientific research has lined up reasons for concern. Studies have linked 2,4-dichlorophenol to problems in aquatic life, including changes to growth and survival. Concentrations above a certain point cause harmful effects in algae and invertebrates that form the base for a whole web of life. In people, research points to liver and kidney stress, headaches, and eye irritation, especially with long exposure or high concentrations.
There’s also a bigger question about how all these low-level contaminants stack up. I’ve noticed this kind of chemical slipping into almost every “urban ecology” case I’ve covered. Most folks don’t get sick from trace amounts, but persistence in rivers and groundwater means you end up with a cocktail no one really planned for. Rural communities, in particular, have raised concerns as runoff affects water wells.
Communities need financial help to test their water, and farmers deserve support to swap out older chemicals for safer ones. Factories ought to treat their waste before discharge. In my own reporting, I’ve watched cities team up with local universities: they run pilot projects using “constructed wetlands” and certain strains of bacteria that eat up 2,4-dichlorophenol before it gets downstream. These solutions work best when they tackle more than one contaminant, since pollution almost never shows up alone.
Transparency matters too. People can make smarter choices and push for change when they know which chemicals might end up in food and water. Pesticide registries and annual water quality reports mean residents see what’s in their tap, not just in dense government bulletins. The more attention 2,4-dichlorophenol receives from watchdogs and communities, the better the odds for cleaner lakes, safer tap water, and fewer nasty surprises in neighborhood backyards.
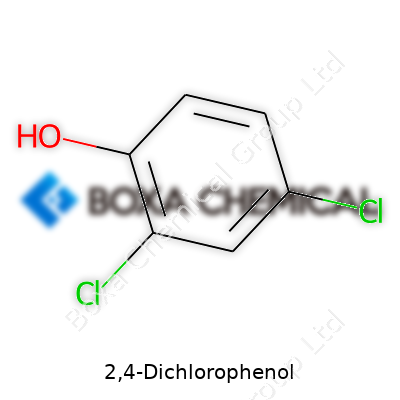
| Names | |
| Preferred IUPAC name | 2,4-dichlorophenol |
| Other names |
2,4-DCP DiCHLORFENOL Dowicide 4 Nurelle D Phenol, 2,4-dichloro- 2,4-Dichloorfenol |
| Pronunciation | /ˌtuː fɔːr daɪˌklɔːrəˈfiːnɒl/ |
| Identifiers | |
| CAS Number | 120-83-2 |
| 3D model (JSmol) | `2DWI/3D` |
| Beilstein Reference | 1209226 |
| ChEBI | CHEBI:1766 |
| ChEMBL | CHEMBL92943 |
| ChemSpider | 8345 |
| DrugBank | DB02628 |
| ECHA InfoCard | 03b8a8b7-7a5c-4712-9e09-3b2e8aa4d164 |
| EC Number | 3.342.170 |
| Gmelin Reference | 82593 |
| KEGG | C02341 |
| MeSH | D004044 |
| PubChem CID | 3440 |
| RTECS number | SN1575000 |
| UNII | 96HN900S0V |
| UN number | UN3438 |
| CompTox Dashboard (EPA) | `DTXSID6020298` |
| Properties | |
| Chemical formula | C6H4Cl2O |
| Molar mass | 163.00 g/mol |
| Appearance | White to yellowish crystals |
| Odor | Phenolic odor |
| Density | 1.381 g/cm³ |
| Solubility in water | 4.5 g/L (25 °C) |
| log P | 2.76 |
| Vapor pressure | 0.005 mmHg (25°C) |
| Acidity (pKa) | 7.9 |
| Basicity (pKb) | 7.41 |
| Magnetic susceptibility (χ) | -73.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.552 |
| Viscosity | 2.7 mPa·s (20 °C) |
| Dipole moment | 2.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 143.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -204.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -824.0 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D08AE06 |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled, causes severe skin burns and eye damage, toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS06, GHS05, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H401 |
| Precautionary statements | P260, P273, P280, P302+P352, P305+P351+P338, P310, P321, P362 |
| NFPA 704 (fire diamond) | 2,4-2 2 0 W |
| Flash point | Flash point: 132°C |
| Autoignition temperature | 700 °C |
| Explosive limits | Explosive limits: 2.2–13% |
| Lethal dose or concentration | LD50 oral rat 580 mg/kg |
| LD50 (median dose) | LD50 (median dose): 580 mg/kg (rat, oral) |
| NIOSH | BZ8575000 |
| PEL (Permissible) | 5 ppm (skin) |
| REL (Recommended) | 0.5 mg/L |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Phenol 2-Chlorophenol 4-Chlorophenol 2,4,6-Trichlorophenol 2,5-Dichlorophenol 2,3-Dichlorophenol |