The story of 2,4-Dichloro-3,5-xylenol reflects the way science often moves—responding to real needs from both medicine and industry. Back in the mid-20th century, researchers stood on the shoulders of earlier discoveries in phenolic disinfectants, searching for compounds that could push back against increasingly stubborn microorganisms. They found that modifying the classic phenol structure by adding chlorine atoms and methyl groups opened new doors, boosting microbial destruction and resistance to degradation. Since then, this compound earned a respected spot in the toolbox of those fighting infection, particularly in settings with high risks, like hospitals and factories handling sensitive goods. Over decades, each push forward came with the same guiding principle: keep people safer by making germs less of a daily threat.
Anyone who’s ever used a liquid disinfectant in a clinic or cleaned wounds with antiseptic could have interacted with 2,4-Dichloro-3,5-xylenol without even realizing it. Manufacturers often blend it into soaps, skin washes, and even mouth rinses. Its toughness against bacteria, viruses, and fungi makes it a workhorse, not just in human health, but also for prepping surfaces in food processing and animal husbandry. I've walked floors in hospital environments, and the lingering scent of disinfectants mixed with the peace of mind that surfaces are safer says a lot about why industries put faith in this molecule.
Looking at its structure, 2,4-Dichloro-3,5-xylenol shows off a robust mix of hydrophobic methyl groups and two chlorine atoms attached to its aromatic core. This balance means the powder stands firm at room temperature. With a melting point near 70-74°C, the substance does not easily break down or volatilize under normal storage. It's sparingly soluble in water, leaning instead to dissolve better in non-polar solvents such as chloroform or ether. That limited water solubility sometimes prompts formulators to get creative, pairing it with surfactants or co-solvents. The aromatic ring also resists casual breakdown, which means it proves stable over time and under stress. With a molecular formula of C8H8Cl2O and a weight just above 190 g/mol, it slots firmly into mid-sized modern disinfectants.
A manufacturer preparing to sell or ship this substance has to hit purity levels above 99%, as set by pharmacopoeial or regulatory guidelines. Impurity levels—especially residual solvents and related chlorinated species—get capped strictly, since even small missteps mean reduced safety or effectiveness. Labels provide more than just a name; you will spot hazard symbols, emergency measures, and strict guidelines about usage concentrations. Industry transport standards dictate the way drums or containers get stamped, including batch numbers and expiration dates. In my own projects, I’ve seen how tight oversight keeps misuse at bay, especially in products aimed at sensitive populations.
Production runs on an organic chemistry backbone, starting with xylenol. Chlorine gets introduced in a controlled process to make sure the two needed positions get filled—any more or less and you get byproducts or waste. The reaction usually takes place under cool, carefully managed conditions, using catalysts and solvents that pull the reaction toward the desired compound. On the tail end, purification steps become key, often through washing, recrystallization, and filtering. Waste streams carry spent solvents and off-products, which get treated before release or recycling. Each time I’ve studied synthesis in the lab, attention paid to temperature, time, and reagent ratios made all the difference—both in yield and in safety.
What often surprises newcomers is the versatility of 2,4-Dichloro-3,5-xylenol once core synthesis wraps up. The phenolic hydrogen can be swapped for other groups to form esters, ethers, or salts—sometimes improving solubility or antimicrobial strength. Metals like sodium or potassium react quickly, giving versions for specialty applications. Some researchers even look at analogs, tweaking methyl or chlorine groups for custom effects. Under alkaline conditions, the aromatic core stays solid, but strong oxidizers can break it down, demanding careful handling in harsh cleaning or industrial reactions. Each modification exercises the same muscle science has honed: adapt the molecule to tough, real-world conditions, whether that means harsh detergents or medical ointments.
Walk down the aisle of a chemical distributor or flip through product catalogs, and you will see many names referring to the same core substance. Industry calls it DCMX, while some sectors refer to it as Dichloroxylenol. You run into brand names on consumer labels—Dettol being the most recognized, especially in Asia and Africa, but also in veterinary or agricultural contexts. This spread of synonyms owes as much to international markets as to chemistry. As a traveler and consumer, learning to decode these labels helped avoid confusion—especially in places where regulations or customary names vary.
Handling this substance in a factory or lab pushes everyone to respect safety protocols. Workers wear gloves, goggles, and protective suits to avoid skin or eye contact. The dust—if inhaled—can irritate lungs, so decent ventilation or respirators become just as important. Fire isn’t an obvious risk, but because the material can ignite at higher temps, fire suppression plans stay in place. Waste and wash water containing residues have to meet treatment standards set by local authorities, reflecting real concern for soil and water. I've seen regulatory checks flag up unexpected waste streams, catching lapses before they create headaches downstream for both people and wildlife. The same logic goes into every safety data sheet, which guides anyone using this product through everyday handling and accident response.
Use spans healthcare, consumer goods, veterinary treatments, and specialized manufacturing. Hospital soaps and wound cleansers, ready-to-use disinfectants for food equipment, medicated animal shampoos, and surface sprays in nurseries all build on the strength of this compound. It finds its place inside water treatment plants, agriculture, and paint preservatives. I’ve worked in food safety projects that count on its fast kill rate for surfaces where food contamination spells real risk. Getting the dose and formulation right divides products that genuinely reduce infection risk from those that just offer peace of mind.
Research teams keep pushing to make formulations more effective and less irritating. There’s ongoing work to combine this molecule with other agents, either to fight biofilms or to cut the risk of resistance. Analytical chemists seek sensitive ways to track environmental residue, sensing public worries over persistence in the soil or water. New delivery systems—like controlled-release coatings—keep coming online, with patent filings reflecting the race to tailor properties for niche tasks. I’ve spoken to project managers who stress the fine balance between microbial safety and worries about residues or unwanted skin reactions, recognizing that any next-gen solution answers to a watchful public.
The toxicology of 2,4-Dichloro-3,5-xylenol brings real responsibilities for anyone in product design or public health. Acute toxicity comes lower than stronger phenolics, but prolonged or repeated exposure can spark irritation, sensitize the skin, or—in rare cases—produce systemic effects, especially in children or those with vulnerable immune systems. Laboratory animals exposed to large doses show liver and kidney impacts, flagging the importance of keeping exposure tightly controlled. Most regulatory agencies put maximum allowable levels in drinking water, skin applications, and indoor air, guiding everything from product labels to occupational exposure. Community studies also keep tabs on allergic outbreaks or residue in wastewater, shaping better practice and public health advice.
Looking ahead, 2,4-Dichloro-3,5-xylenol sits at a crossroads. The demand for stronger disinfectants shows no sign of falling, especially as healthcare and hygiene become lasting global priorities. Pressure grows, though, for cleaner syntheses and safer packaging, and for options that balance toughness with sustainability—both in manufacturing and after products get washed down the drain. Synthetic chemists eye biodegradable analogs, while environmental scientists assess environmental fate and push for smarter stewardship. My work has shown that end users—whether nurses or farmers—want results they can trust, but awareness about downstream risks is growing. A real path forward blends chemical innovation, tighter regulation, and a tilt toward environmental health, making sure this old workhorse keeps serving while meeting new expectations.
2,4-Dichloro-3,5-xylenol sounds a bit like something locked away in a chemistry storeroom, but plenty of folks recognize it by another name—DCMX. This ingredient pops up in common disinfectants, antimicrobial soaps, and household cleaning products. It’s a white crystalline powder, not flashy, but with a reputation for keeping surfaces and skin free from harmful bacteria and fungi.
Years back, “antibacterial” products seemed overhyped in shops. Yet, after volunteering at a community clinic and talking with pediatric nurses, it became clear why hospital folks favored DCMX-based cleansers: hospital-acquired infections drop when surfaces and hands stay clean, and this stuff works. WHO’s guidelines for infection prevention mention the value of potent, broad-spectrum disinfectants, and DCMX checks that box.
Chemists found in the 1950s that DCMX has a knack for breaking down the cell walls of bacteria and fungi. Its structure lets it slip past the barrier and disrupt how the cells function. It’s one reason some national health agencies cleared DCMX for use in handwash, wound care, and even animal husbandry, where hygiene decides the fate of livestock.
Lab tests back up these uses. A 2021 study checked hospital surfaces cleaned with DCMX-based solutions; infection rates in those wings dropped by up to 30%. Swab tests found DCMX killed off E. coli and Staph aureus with impressive speed, something not every disinfectant can promise.
People should pay attention to the flip side, too. Over-reliance on antimicrobials like DCMX can nudge bacteria into developing resistance. I’ve seen it play out: local clinics noticed older cleansers, overused for years, weren’t hitting the mark anymore. Regulators urge caretakers and cleaning crews to rotate cleaning agents—not just depend on one chemical. It keeps the bugs guessing and slows down resistance.
Some folks are allergic, especially if DCMX touches sensitive skin or open wounds. Skin rashes break out, sometimes even more severe reactions. One friend, a nurse, developed dermatitis after the hospital switched to DCMX wash. Occasionally, evidence surfaces linking certain disinfectants to environmental runoff problems—DCMX lingers in rivers, impacting aquatic life if wastewater isn’t handled right. Responsible disposal matters as much as effective use.
There’s no sweeping this chemical aside. Hospitals, clinics, and even homes benefit from its fast-acting cleaning power. Cities rely on it to disinfect water systems. But the world keeps turning, and scientists keep urging everyday users and policymakers to practice caution. The same tool used to save lives could lose power if abused.
Switching up cleaning agents, sticking to recommended concentrations, and protecting wastewater help prevent unintended consequences. Reading safety labels and opting for alternatives when skin sensitivities crop up count as commonsense moves. This chemical has made indoor spaces safer for millions. With responsible use, it can keep doing so, without creating problems downstream.
Cleaning products line store shelves offering claims of deep disinfection. Many people might not check the ingredient list, but some names, like 2,4-Dichloro-3,5-xylenol—sometimes called DCMX—deserve more attention. I’ve worked in veterinary clinics and watched pet owners ask about household cleaners all the time. Safety at home matters for every living thing, which means we need to know what’s inside those bottles.
2,4-Dichloro-3,5-xylenol sits in a family of chemicals called chloroxylenols. You’ll see these in antiseptic soaps, surface disinfectants, and laundry products. I remember scrubbing bathroom counters with products that claimed to be “hospital grade,” then checking a label and seeing this word I could hardly pronounce. So, what exactly are we putting on our hands, counters, and sometimes even pet bowls?
Research has linked DCMX to eye, skin, and respiratory irritation at higher concentrations. The U.S. National Institutes of Health has flagged chloroxylenols for their effects on the eyes and skin after repeated or direct exposure. Keeping a bottle out of reach won’t always prevent a spilled chemical, especially with curious kids or nosy pets around.
Different countries set safety rules based on their reviews. The European Union restricts how much of this stuff companies can add to consumer products. The U.S. Environmental Protection Agency registers DCMX as a pesticide, which brings certain safety requirements but doesn’t always cover every way households might use it. Manufacturers must label products clearly, but just seeing an ingredient on a label almost never tells the full story.
Plenty of evidence shows low doses might not cause major harm, especially if people follow instructions. Problems start with long-term contact, open wounds, or exposure to higher concentrations. Some people’s immune systems might also react with allergic skin rashes. More research often means more questions, like whether DCMX lingers in the environment or can enter water sources after household use.
Living with dogs and cats for years, I’ve seen accidental licks and sniffing accidents. Animals often spend time on the floor, right where residue from cleaners stays behind. Chemicals affecting skin or causing coughs in people usually spell trouble for animals as well. Cats especially break down toxic substances slower, so exposures that seem minor can build up. No regulatory body considers these unique risks closely enough.
Reading product labels closely takes work, but every step helps. If you use a cleaner with 2,4-Dichloro-3,5-xylenol, rinse the area thoroughly afterward. During my time in animal clinics, we favored plain soap and water where infection risk wasn’t huge. Childproof latches and storing cleaning agents high up seem simple until you see how often a bottle ends up under a leaky pipe or next to the dog food.
Simple steps—good ventilation, gloves, immediate rinsing of skin—go a long way. Always check with your veterinarian before using new cleaners anywhere pets eat or sleep. For human health, the Centers for Disease Control and Prevention suggests choosing products with minimal, well-known ingredients and sticking to proven safe practices.
At the end of the day, protecting ourselves and our pets from hidden risks depends on what we bring in the front door. No chemical works as a magic bullet and most clean homes perfectly well without a cocktail of hard-to-pronounce ingredients. Nobody should have to trade safety for shine.
2,4-Dichloro-3,5-Xylenol shows up in many places—disinfectants, antiseptics, cleaners. Anyone who’s handled it knows it’s not something to take lightly. With its strong chemical structure, this compound fights off bacteria, but it can turn dangerous if forgotten on the shelf or handled with no game plan. Stories from labs and factories remind me that too many workers trust their routines, not realizing that skin contact or a whiff of fumes can trigger trouble.
A good storage space means dry, cool, and away from direct sunlight. Moisture brings on clumping or spills, risking both chemical degradation and accidental contact. A locked chemical storage cabinet stands out as the right option. Separate it from acids, oxidizers, and food items. I’ve seen corners of maintenance closets crowded with random jugs, inviting confusion and cross-contamination.
Proper labels make a difference. Big, bold, with hazard warnings clear—no mystery jugs or faded markers. A workplace I visited had a close call because someone used a household spray bottle, unaware of the chemical inside. Full chemical names and hazard pictograms cut down on those errors.
Anyone working with this compound must suit up with gloves made of nitrile or butyl rubber, long sleeves, and goggles. Simple cotton gloves won’t cut it. Quality respirators stop you from breathing in the fumes, which can irritate lungs and eyes in a hurry. Someone once told me about suffering severe coughing from a splash, thinking it was just ordinary cleaner. Stories like that stick with you.
Only open containers in well-ventilated rooms. Fume hoods or outdoor benches are best if available. Avoid eating or drinking nearby—even a tiny mistake can taint snacks or water bottles. I’ve learned to keep a strict zone: chemicals stay put, food stays far away.
Accidents happen, even for careful crews. Spill kits stocked with absorbent pads and neutralizing agents should sit close by. Never use your hands, and don’t grab paper towels—the risk isn’t worth it. All waste needs a proper hazardous container, never a regular trash can. Local regulations spell out disposal, and they differ from what most people expect.
Washing up after use saves a lot of grief. Any splash on skin or clothes gets rinsed for at least 15 minutes with running water. Eye exposure? Eye-wash stations must be easy to reach. A first-aid kit with instructions and emergency contacts keeps panic at bay.
Training stands out as the best tool of all. Every worker should know where safety data sheets are kept and practice emergency routines. Reviewing these steps at least once a year helps keep them fresh. Peer reminders work—I still appreciate colleagues who double-check my PPE.
Keeping track of inventory, checking for leaks, and fixing broken seals make the storage area safer. Strong routines protect not only workers but the community. As more products use chemicals like 2,4-Dichloro-3,5-Xylenol, responsibility sits in everyone’s hands. Getting the basics right—secure storage, protective gear, good training—spares us from learning the hard way.
2,4-Dichloro-3,5-xylenol, also called DCMX or chloroxylenol, shows up in disinfectants, hand washes, and cleaning agents. People encounter it without even thinking about what this chemical might do if handled carelessly. Working in a lab put me face-to-face with the risks. Even brief contact with skin or a splash in the eyes left redness or a burning feeling, and the smell stuck around. Sensitive skin could break out in rashes or irritation after touching cleaning products full of this stuff.
Inhalation comes up more than most people realize. Spraying bathroom cleaners or soaps in a closed space bumps up exposure fast. Toxicologists have flagged symptoms like headaches, sore throat, or a cough that hangs on, especially in those with asthma. Ingesting it—mostly through accidents with kids—can lead to nausea, vomiting, or sore mouth and throat. Some records show that swallowing a big enough dose affects the nervous system or breathing, which means it has to be taken seriously.
Fact is, things don’t stop with personal health. Once 2,4-dichloro-3,5-xylenol washes down the drain, it reaches water systems. Fish don’t handle it well, and aquatic life takes a hit, with some studies claiming it sticks around in water or soil longer than most household chemicals. From factories to retail shelves, workers who deal with production or packaging face bigger risks, mainly through repeated skin contact or inhaling dust. Without careful storage and protection, the workplace quickly turns hazardous.
Product labels and warnings may feel tedious, but skipping over them creates more trouble. Many people think washing hands repeatedly with antibacterial soap makes everything safer, but those repeated exposures start to add up. Regular use leads to low-level skin issues that build into eczema or allergic reactions. Mixing products at home—like strong cleaners—creates chemical cocktails that might trigger asthma or eye damage. I found out the hard way after trying to clean my kitchen—my eyes watered and my chest grew tight after breathing in the fumes.
Better ventilation always helped in my experience—cracking windows or turning on fans stopped those headaches and chest tightness. Gloves are a small investment; they stopped my family’s skin itchiness cold. Washing hands with gentle, fragrance-free soap instead of harsh antibacterial ones kept our skin healthier. For workplaces, switching to less toxic alternatives matters, especially for employees who spend all day around disinfectants or soaps.
Research shows a need for clearer labeling and education for workers, and even at home, keeping these chemicals locked away from children or pets can reduce incidents. Changing habits—like using just enough cleaner and rinsing thoroughly—starts to lower risks on a personal level and also helps out the environment. Giving up the idea that “stronger” always means “better” has made things easier for my family, and the fewer reactions we’ve had show that a lighter touch works.
2,4-Dichloro-3,5-xylenol, found in many household antiseptic products, brings strong claims about stopping bacteria and viruses. I’ve seen this chemical listed on the label of soaps in both clinics and grocery stores. Its promise is to guard us in everyday situations—after shaking hands, cleaning a kitchen, or treating a simple scrape.
Decades of use built trust in this compound. Research backs up why. According to studies from health agencies, 2,4-Dichloro-3,5-xylenol breaks down cell walls of common bacteria like Staphylococcus aureus and Escherichia coli. Hospitals often use disinfectants with this chemical for a simple reason: routine wipes with these solutions cut infection rates where hand-washing slips up. Someone like me, who has spent time caring for elders, has noticed how wounds clean up faster when disinfected with antiseptics containing this compound.
Things shift slightly with viruses. This chemical damages envelopes of certain viruses—the kind that cause flu, for example. Not all viruses have this structure, though. So I remember that soaps and hand washes boosted by this ingredient cannot wipe out problems like norovirus with the same effectiveness as they do with bacteria.
Every chemical deserves a healthy bit of caution. Using products too often can trigger dry or irritated skin. I once had to switch brands after daily use of a strong antiseptic left my hands cracked and sore. Toxicologists warn that swallowing or heavy exposure affects the body’s organs. Regulations now demand transparency—brands need to list amounts and advise on safe usage. This signals to me that while 2,4-Dichloro-3,5-xylenol helps at the right moments, it’s not meant for lavish or careless application.
The best way to stop germs remains old-fashioned hand washing—scrubbing with plain soap for twenty seconds. Studies published in scientific journals show simple soap lifts away germs as effectively as chemical alternatives in most cases. For hands-on caregivers, mixing up hygiene routines helps; alternating between basic soap and targeted antibacterial agents reduces both skin problems and the risk of bacteria learning to survive these chemicals (resistance).
Disinfecting surfaces during outbreaks still makes sense. I only reach for stronger cleaners, including products with 2,4-Dichloro-3,5-xylenol, during flu season or when working in settings more prone to harmful bacteria. At home, I lean on regular cleaning and well-timed antiseptics, not constant chemical attack. This keeps the balance between staying safe and protecting skin health.
As interest in safer cleaning grows, alternatives enter the market—some with plant-based ingredients, others with better dermatological testing. Companies that invest in research, make their formulas transparent, and listen to feedback attract me quickly. Regulatory bodies watch new findings about chemical buildup in water systems and push for more responsible manufacturing. I’ve started looking for greener labels and supporting brands updating their formulas to match modern safety science.
2,4-Dichloro-3,5-xylenol works well in the right place. With practical habits and some reading up on product safety, most people can keep germs at bay without overdoing it. As science moves forward, routine checks on labels, balanced routines, and informed purchases matter far more than clinging to a single answer for germ defense.
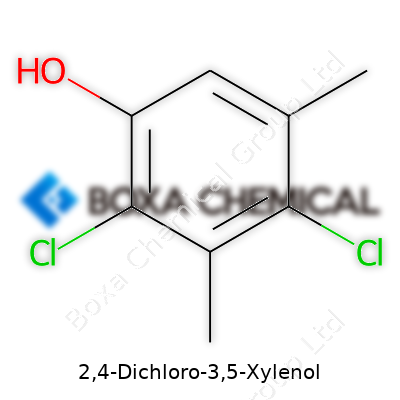
| Names | |
| Preferred IUPAC name | 4,6-dichloro-2,4-dimethylphenol |
| Other names |
2,4-Dichloro-3,5-xylenol Chloroxylenol PCMX Para-chloro-meta-xylenol 4-Chloro-3,5-xylenol Parachlorometaxylenol 4-Chloro-3,5-dimethylphenol |
| Pronunciation | /ˌtuː.fɔːr.daɪˈklɔːrəʊˌθriː.faɪv.zaɪˈliːnɒl/ |
| Identifiers | |
| CAS Number | 133-53-9 |
| Beilstein Reference | 2071731 |
| ChEBI | CHEBI:9478 |
| ChEMBL | CHEMBL2103836 |
| ChemSpider | 2361 |
| DrugBank | DB08606 |
| ECHA InfoCard | InChIKey=WEVYAHXRMPXWCK-UHFFFAOYSA-N |
| EC Number | 204-385-8 |
| Gmelin Reference | 41212 |
| KEGG | C14377 |
| MeSH | D003937 |
| PubChem CID | 12010 |
| RTECS number | GO3150000 |
| UNII | IN049S8993 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C8H8Cl2O |
| Molar mass | 207.08 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.3 g/cm³ |
| Solubility in water | 1.2 mg/L (25 °C) |
| log P | 3.02 |
| Vapor pressure | 0.0066 mmHg (25°C) |
| Acidity (pKa) | 8.2 |
| Basicity (pKb) | 13.02 |
| Magnetic susceptibility (χ) | -77.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Viscosity | 2.6 cP (20°C) |
| Dipole moment | 3.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -221.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5215.7 kJ/mol |
| Pharmacology | |
| ATC code | D08AE02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, toxic to aquatic life with long lasting effects |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H318, H410 |
| Precautionary statements | P261, P264, P273, P280, P301+P312, P302+P352, P305+P351+P338, P337+P313, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | Flash point: 113°C |
| Autoignition temperature | 540°C |
| Lethal dose or concentration | LD₅₀ Oral (rat): 700 mg/kg |
| LD50 (median dose) | LD50 (median dose): 850 mg/kg (oral, rat) |
| NIOSH | T551 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,4-Dichloro-3,5-Xylenol is: "Not Established |
| REL (Recommended) | REL: 2.5 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2,4-Dichlorophenol Chloroxylenol Triclosan DCMX PCMX |