2,4-Di-Tert-Butylphenol emerged decades ago during the growth of the petrochemical industry, when organic chemists leaned heavily on alkylation technology. Early synthetic schemes for substituted phenols often lacked selectivity and safety; stories circulate of glassware shattering as workers raced to moderate runaway reactions. Over time, chemists found more reliable Friedel-Crafts alkylation methods, using catalysts like aluminum chloride and carefully controlling temperature to tame violent exotherms. Despite those early headaches, the pursuit of stable phenolic antioxidants pushed researchers to create this bulky derivative, quickly recognizing how tert-butyl shielding protected the aromatic ring from unwanted oxidation. By the mid-twentieth century, companies folded 2,4-Di-Tert-Butylphenol into their portfolios as a critical intermediate for polymers, rubber, and even specialty agrochemicals, mapping a path from laboratory curiosity to industrial staple.
This compound delivers a punch as an antioxidant, its structure loaded with steric hindrance from tert-butyl groups, almost like bodyguards flanking a VIP. Strictly speaking, it’s an aromatic compound with phenol as the core scaffold and bulky alkyl groups at the 2 and 4 positions. In the lab, flakes or crystalline powder show up in off-white to pale yellow, often carrying a faint odor reminiscent of hydrocarbons. Folks working with food packaging, lubricants, and even adhesives have come to rely on it for stabilizing sensitive formulas. I’ve seen formulators blend this stuff into heat-prone products to delay yellowing and degradation; you can spot the difference with your eyes after weeks in a hot oven. Years of industrial use signal confidence in its stability and ease of blending, but this has never meant it should be handled with complacency.
With a molecular formula of C14H22O and a molar mass just over 206 g/mol, 2,4-Di-Tert-Butylphenol stands out for its low volatility, thanks to those two massive tert-butyl slabs. Its melting point lands close to 53–55 °C; tossing it in a beaker on a cool day means waiting a while before anything starts to flow. The boiling point, well over 280 °C under atmospheric pressure, means handling at higher temperatures requires serious ventilation. Water won’t budge this compound—solubility is so low that environmental releases tend to stick to soils and sediments, not drift downstream. Pour this into non-polar organics, and it dissolves rapidly, which proves handy if you’re adding it to polymer melts or hydraulic fluids. Its phenolic hydroxyl gives it mild acidity, though far weaker than common acids done in aqueous solutions.
Commercial product specs pin down the purity, often above 98%, ringfenced by narrow moisture and ash limits. Careful labeling lists impurities (usually traces of mono-tert-butylphenol or tri-substituted homologs) as well as color, physical form, and packaging details. UN numbers, hazard symbols, and batch identifiers need to be stamped onto containers—no room for mistakes when shipping chemicals with combustion potential. Product labels often show the CAS number, 96-76-4, and extended hazard phrases, urging users to avoid inhalation or skin contact. MSDS sheets fill out the backstory, breaking down flash point information and suggested PPE like splash goggles and nitrile gloves. Traceability reigns as a core principle, not just for legal compliance but because a contaminated batch in rubber manufacture can cost millions if tires fail the rolling test.
The go-to path for making this phenol runs through Friedel–Crafts alkylation, as old-school as organic chemistry itself. Starting with phenol, industry turns to isobutylene or tert-butyl chloride as alkyl donors, and aluminum chloride as the Lewis acid catalyst. Operators charge reactors with phenol under dry, cooled conditions, drip in the alkyl donor, and keep an eagle eye on exotherms that could force an emergency quench. HCl gas vents off and must be scrubbed; people working these batch reactions learn to respect the pressure gauges. Once the reaction eases, mixtures run through aqueous work-up and neutralization. Distillation finishes the job, isolating the pure product from leftover reagents. Advances in green chemistry push process engineers to swap in less corrosive catalysts—zeolites, ionic liquids, and even solid acids are getting a look these days, pointing toward safer, more sustainable production models.
This molecule doesn’t go quietly into most reactions. Thanks to tert-butyl bulwarks, electrophilic attack crawls to a halt at the aromatic ring, but the phenolic OH keeps things interesting. Acylation, etherification, or coupling with diazonium salts spins out a web of derivatives used in specialty applications. Under basic or reducing conditions, the core ring sometimes cracks open, giving birth to fragmented products with their own quirks. I’ve seen chemists shoot for more exotic analogs by swapping the tert-butyl groups for other branched alkyls, hoping to fine-tune steric effects. Its oxidative stability means you won’t catch it breaking down during storage, a trait treasured by manufacturers who can’t afford downtime for resin replacements. Some researchers experiment with cross-coupling strategies to anchor this core into larger frameworks, chasing new antioxidants and stabilizers for cutting-edge materials and pharmaceuticals.
Walk past the catalogs and listings—this compound wears several aliases. Some know it as “2,4-DTBP,” others as “Fenoxan” or “Kramox 50.” Regulatory paperwork sometimes calls it “Phenol, 2,4-bis(1,1-dimethylethyl)-,” a mouthful if I ever saw one. Catching familiar patterns in product codes helps trace suppliers, but these synonyms can trick the unwary during purchasing. Some regional markets favor one name, and you quickly discover mistakes can tangle legal compliance or disrupt supply chains. It pays to lock down standardized labeling and always cross-check the CAS number, since even similar-sounding chemicals can have wildly different safety profiles.
Handling this stuff, you get an up-close lesson in chemical hygiene. Direct skin contact brings irritation, so long sleeves and gloves become second nature. Inhaling dust or vapor leads to headaches and throat discomfort, sometimes more severe outcomes with repeated exposure. The compound carries moderate toxicity—animal studies report LD50s low enough to command respect in the plant. Spills on hard surfaces turn slick and stubborn; cleaning crews need anti-static brushes and suitable absorbents to avoid contamination. Storage calls for tight-sealed drums under cool, dry conditions, away from acids and oxidizers. I’ve heard of careless stacking warping drums, a nightmare if contents begin leaking. Fire hazards get a major spotlight during audits—flash points mean special extinguishers and dedicated containment, since a warehouse fire can wipe out stock and threaten neighborhoods. OSHA and REACH regulations carve out mandatory air quality monitoring and emergency showers, no exceptions.
2,4-Di-Tert-Butylphenol enters into diverse workspaces. Antioxidants for rubbers and plastics account for a huge slice, especially where heat and light beat on products—think insulation foams, wire sheaths, and automotive interiors. Food packaging leverages this molecule to shield polymers from decomposing under sterilization cycles, though purity must hit tight marks to meet food contact approval. Lubricant formulators chase longevity for engine and hydraulic oils, counting on this phenol’s ability to stymie sludge formation. In agriculture, it acts as a building block for herbicides and other crop protection agents, engineered for slow degradation in soils. Shifts in environmental standards mean more research into alternatives, but 2,4-Di-Tert-Butylphenol clings to ubiquity because legacy equipment, supply agreements, and product registrations often move slower than innovation. Functional coatings and barrier films for electronics also depend on this compound to block air, moisture, and chemical attacks, improving reliability in harsh settings.
Academic labs and industrial R&D outfits keep probing new corners of this compound. In the race for greener chemistry, there’s a surge in work on catalytic systems that promise lower waste—a shift from mineral acids to solid-phase versions and designer ionic liquids. Analytical chemists dig through degradation pathways, searching for marker compounds that help trace environmental contamination. There’s ongoing effort to design derivatives with tailored performance, fitting improved solubility, lower toxicity, or better synergy with other stabilizers. As electronic devices slim down, formulators study this material’s dielectric properties, hoping to push the limits of miniaturized circuit boards and flexible displays. Supply chain uncertainty sends some teams into the field, qualifying biobased sources for key reagents. Inevitably, regulation spurs more toxicological screening, driving both product innovation and safer alternatives, particularly as older stabilization methods draw greater scrutiny from health authorities.
Years of toxicity research show a nuanced picture. Acute oral exposure studies in rodents set the LD50 in the range of 1600–2000 mg/kg—low enough to treat this with caution, though not “drop dead at a whiff” territory. Mutagenicity screens turn up negative, but chronic dosing brings some signs of liver or kidney stress. Environmental fate research says this compound sticks tightly to soils, where microorganisms break it down slowly, a trait that prolongs its footprint but lessens mobility. Waterway spills raise alarms, as even modest concentrations linger in sediments and bioaccumulate in aquatic species over weeks or months. I’ve seen wastewater treatment protocols tuned to accelerate removal, but this calls for activated carbon or advanced oxidation. Respiratory safety is non-negotiable— chronic inhalation could fuel allergies or lung inflammation, so robust ventilation and monitoring rank high for anyone routinely working with this chemical. Increasing interest in “safe-by-design” policies means every data gap gets filled in, either by new studies or predictive toxicology modeling, as part of staying compliant with stricter international directives.
2,4-Di-Tert-Butylphenol holds onto its position, but faces both challenges and opportunities. Proven antioxidant performance gives it durability, but four big forces lean on its future. First, regulatory watchfulness grows sharper, especially in Europe and North America where authorities eye phenolic derivatives for potential endocrine and environmental risks. Second, industry looks hard at its carbon footprint—pressure on petroleum-based chemicals fuels a hunt for renewable analogs, sometimes blending or replacing this compound to meet evolving eco-label requirements. Advances in catalytic process design promise cleaner manufacturing routes and lower waste, opening the door to cost savings and green marketing claims. Material scientists push the envelope on stability and performance, demanding new derivatives, so research money flows elsewhere if innovation stalls. Up-and-coming regions in Asia and Latin America grow their local capacity, seeking to cut costs, but this triggers intense scrutiny of quality controls and toxicology. Solutions lie in transparent supply chains, ongoing worker safety training, and relentless technical innovation so products outperform rivals on safety, not just price.
2,4-Di-Tert-Butylphenol pops up across different fields — pesticides, plastics, and even medicine. You probably won’t find a bottle of it in someone’s garage, but this compound shows up in many products folks use every day. Its name is a handful, but it’s all about those two bulky tert-butyl groups attached to a phenol core. That structure gives it some unique strength: stopping things from breaking down or going rancid.
Think about food packaging, insulation foam, car dashboards. Sunlight and heat wear these down over time, turning useful plastic into cracked, crumbly mess. Chemicals like 2,4-Di-Tert-Butylphenol slow fading and extend the life of those materials. It acts as an antioxidant, meaning it grabs up free radicals before those rough molecules start chains of damage. Instead of tossing faded plastic every few years, this helps products hang in there much longer.
Ever notice how fruit and grain crops get moldy or rot when fungus moves in? Farmers use blends of protectants, and sometimes formulations call for 2,4-Di-Tert-Butylphenol. This compound stops fungus from spreading, at least according to several studies in agricultural science. Even some medical research points out that it has anti-microbial and anti-fungal abilities — which means it holds promise far beyond spraying on a field. Of course, nobody wants to overdo chemical exposure in food, so regulators monitor levels carefully.
Researchers figured out that those big tert-butyl groups make the molecule hard for most living things to break down. The phenol itself can react with unstable cellular molecules, mainly peroxide and free radicals. This disrupts the chain reactions involved in spoiling foods or causing materials to break apart. The antioxidant game isn’t just about longer shelf lives, though. There’s also interest in medicine: some studies show the chemical slows certain bacteria and fungi in clinical cultures.
On paper, a chemical like this looks promising — it does a job and can be used in small amounts. In real life, scientists don’t just chase performance. There’s talk about byproducts, long-term residues, and the need to keep food and water safe. The takeaway: balance is everything. Regulatory groups like the EPA and EFSA want to see proof about levels in finished goods and how much risk lands on people or the environment. In the past, ignoring these questions led to huge problems with older, more toxic additives.
If you work in industry or even care about quality in products, chemicals like 2,4-Di-Tert-Butylphenol become part of the bigger debate. Companies and scientists have a job: figure out ways to use the smallest effective amount, keep things out of landfills, and share safety info. For folks outside the lab, it’s good to know these molecules are part of keeping stuff fresher and safer — even if the best future might include better, greener alternatives as research moves forward.
Every day, folks interact with chemicals at work, at home, and out in the environment. You might not see them, but they’re there: cleaning products, plastics, even the air. 2,4-Di-tert-butylphenol, often shortened to DTBP, stands among the thousands of compounds used in industry. Recognizing the impact of something like DTBP on health does not call for a science background—it only takes care and a willingness to learn the facts.
DTBP finds its way into products for a simple reason: it acts as an antioxidant. That means it helps slow the breakdown of some plastics, rubbers, and fuels. Its job sounds invisible and, in a lot of places, it is. Those working in manufacturing might breathe in fumes or get DTBP on their skin. Most people, though, won’t ever know they crossed paths with it.
Exposure to DTBP by inhaling dust or through skin sometimes bothers people. Studies on animals have signaled potential links to irritation and organ effects at high doses. No one has stepped forward with evidence tying normal background exposure in daily life to long-term problems. Still, that doesn't mean the coast is clear. The European Chemicals Agency flagged DTBP as hazardous due to possible risks to reproduction and environmental effects. This brought me back years ago, working a job that required gloves and masks on the factory floor. Little irritations from chemical dust turned into coughs, itchy skin, and, some days, headaches that did not quit. It opened my eyes—risk can sneak past in tiny doses, especially for folks working directly with chemicals like DTBP.
Authorities review new health information as research comes in. In Europe and the U.S., regulators encourage companies to substitute safer alternatives if there’s cause for concern. Workers in chemical plants use protective gear for a reason: gloves, goggles, and air filters don’t get handed out just to look good. Personal experience taught me that skipping simple protection with chemicals, even the ones said to be safe, has real consequences. If a lab coat and gloves sit on the hook by the door, it’s smart to put them on—company policies exist to protect people, not slow them down.
For most people, respect for chemical safety means following product instructions and storing things safely. I learned early on that even a single spilled bottle in a household cabinet can cause problems for kids or pets. At work, insist on training and proper labeling. Refuse to take shortcuts—safe practices matter just as much as deadlines. If there’s ever doubt or concern about a particular chemical, asking a supervisor or safety officer puts everyone in a stronger position.
No substance carries zero risk when handled carelessly. Science may not have nailed down all the long-term impacts of DTBP, but steps to protect workers and the public make a difference. I’ve seen sites where fresh air, smart rules, and open communication helped people avoid illness, even while surrounded by industrial chemicals. Staying informed, asking questions, and choosing caution takes little effort and pays off down the line.
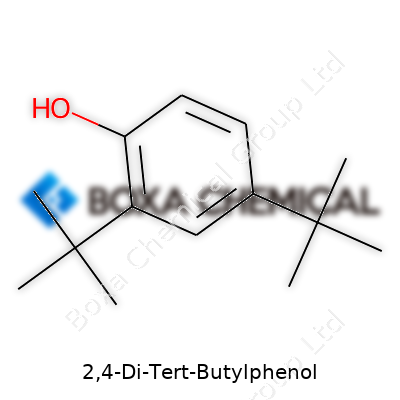
Chemistry often rewards patience. To reach the molecular formula of 2,4-Di-Tert-Butylphenol, it helps to have hands-on experience tackling organic structures. The compound starts with phenol at its core – a benzene ring bridged by a hydroxyl group. Two tert-butyl groups find their place at the second and fourth positions on this ring. Tert-butyl, recognized in countless labs for its bulkiness, brings along three methyl groups and a central carbon, swelling the molecule’s footprint quicker than many other substituents.
Let’s translate these pieces into actual atoms. The phenol ring includes six carbons from benzene and one from the hydroxyl group’s oxygen. Each tert-butyl group holds four more carbons (one central, three methyl) and nine hydrogens. Two of those, and the phenol core, means:
Total it up: 6 (ring) + 8 (side chains) = 14 carbons. Hydrogens add up as 18 from tert-butyls plus those attached where substitutions happen—this real-world calculation always makes me remember a wrinkled textbook corner from college, cheddar cheese stains included, fixing an error just like this one. Ensuring each substituent lands on the right carbon to avoid overcounting gets easier once you’ve misassigned one or two yourself.
After double-checking, we find:
That leads straight to the formula: C14H22O.
I’ve seen this molecule give headaches in synthesis, not from its structure but from the confusion that can swirl around its derivatives. Students and new chemists sometimes overlook the importance of double-checking formulas before mixing reagents or interpreting analytical data. Getting the molecular formula right doesn’t just help on exams; it sets up accurate calculations for molarity, helps with NMR analysis, and even protects against scaling errors when working with larger batches.
Just last year, during a small-scale antioxidant screening project, the team almost miscalculated dilution ratios due to misreading the formula of 2,4-Di-Tert-Butylphenol. Careful review of the chemical’s structure and formula prevented both wasted effort and potentially dangerous concentrations.
Pushing for clearer visual aids in textbooks and lab manuals would help. Digital tools now quickly draw structures based on names and spit out formulas, but not everyone trusts or uses them. Hands-on drawing and peer review in academic settings bring down error rates. In industry, integrating structure-validation software into procurement and synthesis design reduces both cost and risk.
Chemical safety hinges on small details. Getting the molecular formula of 2,4-Di-Tert-Butylphenol right—C14H22O—reflects a larger discipline: attention, vigilance, and open discussion in both learning and practice. It’s worth more than a right answer; it’s a way to help science move safely and reliably.
2,4-Di-Tert-Butylphenol may sound like a tongue twister from a chemistry class, but this chemical turns up in labs and factories around the globe. It acts as an antioxidant and stabilizer for plastics, rubbers, and fuels. With steady use comes the need for proper storage, not just for the folks on the front lines, but for the safety of everyone nearby.
Reading safety data sheets is a good starting point, but real-world storage needs more than a checklist. I’ve run into people who think chemicals like this are safe to leave on a shelf, just a bit out of reach. That’s a mistake. Mishandling leads to skin irritation, respiratory problems, and environmental damage if it leaks.
Temperature tells much of the story here. 2,4-Di-Tert-Butylphenol doesn’t play well with heat. Too much warmth pushes its vapors into the air. Pretty soon, stuff that starts as an antioxidant becomes an unpredictable hazard. Any space chosen for storage must stay well below room temperature—think cool basements or ventilated storage rooms.
Real-life example: in one small factory, staff stored a similar phenol compound by a steam pipe. The chemical vaporized more quickly, and workers started coughing before realizing the problem. They moved the stock to a dedicated ventilated area, and symptoms disappeared. Ventilation cannot be ignored. Clean airflow prevents dangerous fumes from hanging around, especially in closed spaces.
Light and humidity punch right through standard packaging. Sunlight degrades most chemicals, and 2,4-Di-Tert-Butylphenol isn’t an exception. Moisture seeps in, triggering slow chemical changes. Chemical barrels or jars must always live out of the sun in a dry, covered space.
People sometimes overlook the basics. Every container holding 2,4-Di-Tert-Butylphenol should carry a clear, bold label. In my days handling chemical stores, at least once a month, someone asked, “What’s in this drum?” Labels stop confusion and accidents. Seals must hold strong. Once, a loose drum cap let fumes escape in a back storeroom, and it wasn’t long before a sharp smell tipped us off to the mistake.
2,4-Di-Tert-Butylphenol doesn’t like living next to acids, oxidizers, or anything flammable. One poorly planned layout can turn a minor spill into a full-blown emergency. In places I’ve worked, rows of yellow warning tape and locked storage cages set boundaries. It might seem overdone, but one chemical reacting with another can ruin more than your day.
Spills happen, no matter how well you plan. Everyone who works near this chemical should have gloves, goggles, and spill kits within arm’s reach. It’s not about paranoia—it’s about being ready. Training drills mean employees know how to handle leaks without guessing.
Safe practices around 2,4-Di-Tert-Butylphenol cut risks at every point, from the storage room to the loading dock. Storage is often an afterthought, but the right habits prevent harm, protect the workforce, and keep the environment safe from accidental releases. Time spent planning proper storage is always worth the effort.
2,4-Di-Tert-Butylphenol shows up on lists of industrial phenols that can cause harm without the right precautions. It looks harmless at first glance—a colorless to light yellow liquid or solid—but safety takes more than reading a label. Skin, eyes, and organs all react badly to carelessness around this stuff. Even common accidents like splashes and spills create real risks in both labs and plants. Experience shows that simple gear often makes the difference between a routine shift and a trip to the emergency room.
No shortcuts with gloves here. I reach for nitrile or neoprene gloves, because latex doesn’t hold up against strong organic chemicals. Lab coats and closed footwear matter, but safety goggles might save your sight—one drop in the eye means a painful chemical burn. When pouring or handling bigger amounts, a face shield adds another layer. Respiratory protection comes out in any setting with poor airflow. Always check the Safety Data Sheet for what meets the exposure limits, but most folks I know in the field recommend the classic N95 for low concentrations and heavier protection if the air gets thick.
Ventilation isn’t just a box to check. I stick to fume hoods or well-ventilated labs so fumes don’t build up. No eating or drinking in these areas, because accidental hand-to-mouth transfer stays a leading culprit for chemical exposure. I keep all containers clearly labeled and tightly sealed, since volatile compounds turn the workplace risky in a hurry.
Chemical spills always teach the same lesson. Speed and knowledge matter. I mop up small splashes right away, using absorbent pads or sand, never hosing it down a drain. Then the waste goes into a dedicated hazardous collection bin. Larger spills, or exposure incidents, call for the site’s emergency protocol. Everyone should know where the eyewash and safety showers are. I’ve watched colleagues waste valuable seconds because they weren’t prepared, and sometimes seconds count.
Storing this chemical away from sources of heat or direct sunlight keeps it stable. Flames, sparks, and static electricity don’t mix with volatile phenols. Sturdy shelves at eye level reduce the risk of breakage. I’ve seen leaky lids cause headaches, literally, because vapors slipped out overnight. Anyone working late could have walked right into invisible danger.
Training comes up a lot in chemical safety conversations. It’s not just about paperwork—real practice with PPE, cleanup, and emergency protocols forms the backbone of lab safety. Even a seasoned technician benefits from a refresher. Good notes can save trouble if issues crop up later, because managers and health teams need all the facts for cleanup or medical care. Regulations may come from OSHA or local agencies, but in my time I’ve seen individual responsibility drive safer labs as much as law. It’s about protecting your coworkers and yourself. Mistakes with chemicals like 2,4-Di-Tert-Butylphenol stick around much longer than the end of a shift.
The best labs and plants reduce unnecessary contact with hazardous chemicals. Automation, whether by closed systems or remote equipment, keeps people out of harm’s way. Substituting less hazardous chemicals if possible, or minimizing the amount used, adds another layer of defense. I encourage even junior staff to speak up if a process looks risky. A quick talk now prevents a big problem later. Knowledge, vigilance, and basic habits always carry more weight than luck when dealing with phenols.
| Names | |
| Preferred IUPAC name | 2,4-di-tert-butylphenol |
| Other names |
2,4-DTBP 2,4-Di-tert-butylphenol DTBP Phenol, 2,4-di-tert-butyl- 2,4-Bis(1,1-dimethylethyl)phenol |
| Pronunciation | /tuː fɔːr daɪ tɜːrt bjuːtɪl fiːnɒl/ |
| Identifiers | |
| CAS Number | 96-76-4 |
| 3D model (JSmol) | `3D_F12[H]Oc1ccc(C(C)(C)C)cc1C(C)(C)C` |
| Beilstein Reference | 635867 |
| ChEBI | CHEBI:34617 |
| ChEMBL | CHEMBL14260 |
| ChemSpider | 14509 |
| DrugBank | DB13857 |
| ECHA InfoCard | ECHA InfoCard: 100.009.216 |
| EC Number | 201-823-0 |
| Gmelin Reference | 119174 |
| KEGG | C06536 |
| MeSH | D000068291 |
| PubChem CID | 3427 |
| RTECS number | SJ8575000 |
| UNII | 19JRX6545P |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | `DTXSID5020699` |
| Properties | |
| Chemical formula | C14H22O |
| Molar mass | 206.32 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | phenolic |
| Density | 0.889 g/cm3 |
| Solubility in water | Insoluble |
| log P | 3.6 |
| Vapor pressure | 0.000102 mmHg (25°C) |
| Acidity (pKa) | 15.0 |
| Basicity (pKb) | 7.89 |
| Magnetic susceptibility (χ) | -45.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.052 |
| Viscosity | 11.6 mPa·s (25 °C) |
| Dipole moment | 2.79 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 175.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -389.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -6540.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, toxic to aquatic life with long lasting effects |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H411 |
| Precautionary statements | P261, P273, P280, P301+P312, P305+P351+P338 |
| NFPA 704 (fire diamond) | 2,4,0,0 |
| Flash point | 113 °C |
| Autoignition temperature | 510°C |
| Explosive limits | Lower: 1%, Upper: 7% |
| Lethal dose or concentration | LD50 (oral, rat) 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3300 mg/kg (rat, oral) |
| NIOSH | KN1575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,4-Di-Tert-Butylphenol: Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Phenol 2,6-Di-tert-butylphenol 4-tert-Butylphenol 2-tert-Butylphenol 2,4,6-Tri-tert-butylphenol |