The journey of 2,4,5-Trimethylphenol runs parallel to the growth of synthetic organic chemistry through the 20th century. Early phenol derivatives grabbed the attention of chemists looking for new molecules that could push the boundaries of dyes, plastics, and chemical manufacturing. Once chemists learned to tweak the methylation of phenolic rings, this trimethylphenol variant emerged from work on coal tar distillation and alkylation reactions. Over decades, improvements in purification and large-scale synthesis helped labs transition from gram-scale curiosity to multi-ton batches fitting for industrial needs.
2,4,5-Trimethylphenol stands out in specialty chemicals due to its unique arrangement of methyl groups around the phenol core. The structure shapes how this compound behaves in both simple extractions and advanced modification reactions. Many companies source it as a colorless or faintly yellow liquid or crystalline solid. Its presence threads through the supply chain of antioxidants, resins, and certain agrochemicals, often serving as a precursor rather than an end-use product. With increased scrutiny on supply chain traceability, the handling and origin documentation of intermediates like 2,4,5-Trimethylphenol have become standard practice in both procurement and R&D.
2,4,5-Trimethylphenol carries a molecular formula of C9H12O and a molecular weight nearing 136.19 g/mol. Its melting point usually falls between 50 to 54 degrees Celsius, and it boils just shy of 240 degrees Celsius. From working with this compound, its characteristic phenolic odor is hard to miss—most lab technicians associate it with a sharpness accompanying ortho-alkylated ring systems. The solubility favors organic solvents but fights back against water, consistent with other methylated phenols. Stability remains respectable under ambient temperature; heat and oxidizers start to degrade the molecule.
Producers typically set purity levels above 98% for industrial customers and over 99% for analytical grades. Color, acidity, and residue levels all attract scrutiny in quality assurance protocols. A standard container wears hazard pictograms, GHS-compliant warnings, CAS number (to identify 527-60-6), and detailed batch information for traceability. Handling guidelines on the MSDS sheet list standard PPE (gloves, eye protection) alongside spill response measures and ventilation recommendations. Import regulations vary by country but often demand accurate labeling for customs and workplace safety authorities.
Most companies rely on methylation of xylenols (such as 2,4-dimethylphenol) with methylating agents like methyl iodide or dimethyl sulfate, often using Friedel–Crafts conditions to push the third methyl group into the 5-position efficiently. The process needs precise temperature control and close monitoring. Any shortcut in catalyst handling or solvent selection can affect both yield and purity. Post-reaction, steam distillation and vacuum crystallization separate product from by-products such as unreacted reactants, higher methylated phenols, and tarry residues. The process generates both chemical and physical hazards, making process engineering as vital as synthetic strategy.
Chemists often target 2,4,5-Trimethylphenol for further functionalization. The methyl groups create a mix of steric hindrance and electron-donating effects, changing both regioselectivity and reactivity in classic phenolic reactions. Nitration, halogenation, and alkylation become more controlled, multiple routes open up for creating pharmaceutical intermediates, advanced resins, and specialized antioxidants. Demethylation or etherification can shift the base molecule toward custom applications with tailored electronic properties. Over-oxidation risks ring opening, forcing teams to carefully balance conditions.
Beyond its IUPAC designation, you’ll find this molecule cataloged as Sym-TMP, sym-Trimethylphenol, and sometimes as Timol A in company brochures. Tradenames tend to hint at source or purity (such as “Trimexol-245” or “Phenolix-2,4,5”), depending on the manufacturer’s branding. Synonyms play a practical role when searching SDS databases or comparing historical literature, as older patents and journals often use a naming convention that predates standardized numbering.
Handling 2,4,5-Trimethylphenol brings familiar risks encountered in laboratories and manufacturing plants working with volatile aromatics. It can irritate skin, eyes, and respiratory tract, with the phenolic nature escalating risk of burns or allergic responses. Chronic exposure, even at low concentrations, demands robust engineering controls, personal protective equipment, and rigorous training. Facilities move toward closed-loop systems and automated handling to minimize worker exposure. Modern EHS guidelines push beyond direct safety—air monitoring, regular audits, and real-time sensor technology keep operations accountable and transparent. This level of diligence reflects both the letter and the spirit of sector regulations.
2,4,5-Trimethylphenol has carved out a space in manufacturing antioxidants used for stabilizing plastics and rubber. Some downstream labs turn it into fragrance chemicals, while others tweak its ring to serve as a building block for crop-protection products or specialty pharmaceuticals. Research into electronic materials, such as new classes of resins, also circles back to the unique substitution pattern of this molecule. Strict product specifications and customer-driven performance requirements shape which applications continue to flourish, with feedback cycles between users and suppliers shaping future developments.
Academic interest in 2,4,5-Trimethylphenol runs deep. Chemists study the interplay between steric bulk and electron-donating effects of methyl groups, often extending findings toward new catalysts or eco-friendly reaction pathways. Recent years have seen intensified work on greener synthesis routes, including alternative methylating agents and bio-based feedstocks. Multidisciplinary teams—from toxicologists to process engineers—keep raising the bar for both synthetic efficiency and downstream compatibility. Computational modeling now accelerates structure-activity relationship studies, sharpening the focus on real-world outcomes rather than theoretical speculation.
Toxicologists dug into the acute and chronic toxicity of methylphenol isomers, especially with the growing role of this compound in specialty formulations. Acute exposures trigger rapid skin or respiratory irritation, and the compound can pass quickly through cell membranes, influencing CNS activity in animal models. No major mutagenic or carcinogenic signals have surfaced in the mainstream literature, but regulatory agencies keep evaluating data from long-term industry and academic studies. Waste management often dominates safety discussions, as improper disposal leads to environmental contamination that lingers for years. Strict guidelines form the backbone of workplace limits and local discharge permits.
Synthetic chemistry and materials science keep opening doors for the next generation of phenolic derivatives, and 2,4,5-Trimethylphenol remains part of the toolkit. The shift toward green chemistry holds promise for lowering both cost and environmental impact through biocatalysis and renewable feedstocks. Broader global interest in circular economy principles—closed-loop manufacturing, solvent recycling, and zero-waste synthesis—promises to change how both manufacturers and customers think about legacy chemicals and their by-products. As researchers map structure-property relationships, opportunities for smart polymer additives, specialized agrochemicals, and even renewable energy components come into clearer focus. Scrutiny from health and environmental advocates ensures companies keep innovating across both product and process. Lab and plant workers, supported by advanced monitoring and transparent reporting, now form the frontline in turning industry best practices into new standards.
Folks who don’t work in a lab probably haven’t heard of 2,4,5-Trimethylphenol. Even so, this chemical has a story that’s worth exploring, especially if you ever care about how common things like antiseptics, dyes, or plastics turn out the way they do. Chemists treat it as more than a name on a bottle—it ends up shaping major products behind the scenes.
For anyone thinking that this compound just sits around, the opposite is true. 2,4,5-Trimethylphenol helps drive the production of antioxidants and some medicines. You’ll find it used in the laboratory as a building block. Its unique structure, featuring three methyl groups stuck to a phenol ring, makes it handy for creating downstream chemicals—compounds you won’t see on a supermarket shelf, but you trust that scientists have put together for a good reason. The phenol part offers natural reactive power for chemical reactions, while the extra methyls change how things react. This lets researchers make complex molecules, including special plastics and resins, that show up in the products people use every day.
Most folks have touched items made using tough, long-lasting plastics. The trick is, those plastics can break down when exposed to sunlight, heat, or oxygen. 2,4,5-Trimethylphenol helps slow down those breakdown processes. By going into certain antioxidant formulations, this compound keeps things like rubber, lubricants, and industrial oils from getting brittle or turning yellow. A strong antioxidant gives plastics that reliable feel and look that people expect, boosting both their safety and how long they last on store shelves or factory floors.
Beyond plastics, this chemical also shapes the world of dyes and pigments. Take printing inks or paints used on metal and wood—those rich, stable colors don’t just happen. Manufacturers often pick key molecules derived from 2,4,5-Trimethylphenol, since they hold their color longer and resist fading from sun or weather. In a world where bright red bumper paint or a magazine cover color actually stands out, this kind of science keeps things vibrant and durable.
You might not swallow pills straight from a chemical vat, but compounds like 2,4,5-Trimethylphenol show up in the making of a few important drug ingredients. Chemistry teams use it as a starting piece for more advanced molecules that wind up controlling pain or fighting infections. Even with tight regulation in the medical world, this compound has secured a reputation for value in research and production.
Mishandling strong chemicals always comes with risk. Researchers follow strict protocols for storage, use gloves, and use ventilated hoods to prevent overexposure. People making or handling 2,4,5-Trimethylphenol don’t treat safety as an afterthought. Those who test for toxicity and track environmental impact have helped create clear guidelines, so the chemical’s benefits don’t tip into causing harm.
Efficiency remains a focus, as companies look for ways to reduce waste and limit exposure to hazardous materials. Teams explore “greener” pathways for making needed molecules, and keep recycling or safer substitutions in the mix. Still, as long as people want longer-lasting plastics, sharper colors, and reliable drug synthesis, expect 2,4,5-Trimethylphenol to keep getting called off the bench. Its place in industry reminds us how smart, careful chemistry continues to touch daily life.
I’ve always found that learning about chemicals yields insights into both their benefits and risks. 2,4,5-Trimethylphenol, a compound tucked inside the family of methylated phenols, shows up in places that might surprise you, from specialty chemical manufacturing to refining certain flavors and fragrances. Its unmistakable aroma often reminds people of creosote or freshly treated wood, so the sensory impact isn't hard to miss. This molecule doesn’t just smell unique; its structure and behavior under everyday conditions reveal why it stands out in a crowded field of phenolic compounds.
With its chemical framework—a benzene ring holding three methyl groups at positions 2, 4, and 5 along with a hydroxyl group—it forms squat, white starting crystals at room temperature. It likes to settle into a solid state between 69 and 74 degrees Celsius, so it can be shipped and handled more safely compared to more volatile organic compounds. It doesn’t float on water, but sports a higher density than many organic solvents—resting around 1.04 grams per cubic centimeter—which tells handlers to consider both environmental and containment planning during spills.
It barely dissolves in cold water, but finds a home in most organic solvents—ether, acetone, and chloroform treat it kindly. If you’ve spent time in a lab, you’ll recognize that this pattern spells both good extraction potential and the need for careful ventilation. For industries or researchers handling this substance, the low vapor pressure at standard room conditions keeps things safer. You won’t see it racing to fill the air with fumes, compared to solvents like benzene or toluene. Still, given its pungent odor, a small release is easy to notice.
This trimethylphenol doesn’t just sit by during chemical reactions. The three methyl groups crowd the ring and influence how it reacts with acids, bases, and oxidizing agents. The hydroxyl group on the ring acts as a signal flare, making it much easier for the molecule to donate a proton compared to typical alcohols—one small reason why phenols carry both respected and risky reputations in industrial settings.
I once worked with a research team breaking down complex organic residues from wood tar, and 2,4,5-trimethylphenol’s stability against oxidation compared to other phenols quickly became apparent. Under mild conditions, it stands up to oxidants, but stronger agents will force it to break down and release potentially hazardous byproducts. For chemical manufacturers, understanding how far it can be pushed before decomposing isn’t academic—it’s a matter of worker safety and environmental compliance. Chemists can methylate phenol selectively to produce this compound, but the yield and purity depend on careful control of temperature and reagents.
Exposure to 2,4,5-trimethylphenol usually prompts advice from occupational health experts: minimize skin contact, provide goggles, keep air movement steady. Its potential for irritation, especially via inhalation or prolonged contact, can’t be ignored, no matter how familiar it smells. Regulatory agencies, including the US EPA and EU REACH, require clear labeling, safe storage away from strong bases and oxidizers, and effective spill cleanup protocols. Disposal routes follow strict hazardous waste guidelines. Fortunately, as long as makers and users keep to established limits, cases of acute poisoning or chronic exposure remain rare.
For anyone in the supply chain—producer, transporter, downstream user—a thorough understanding of material safety data, strong investment in engineering controls, and dedicated safety training all matter. Real progress often comes when teams treat chemical management as an ongoing dialogue, adjusting protocols during near-misses, and seeking greener alternatives whenever efficiency and cost allow. Investing in better ventilation and spill response equipment pays dividends over time, both for worker health and for local communities worried about chemical runoff. With 2,4,5-trimethylphenol, the details really do make the difference.
2,4,5-Trimethylphenol turns up mostly in labs and industries tied to chemical production and synthesis. It isn’t sold on store shelves for everyday consumer use. I remember coming across information about this compound in industrial safety training sessions, usually in the context of handling raw materials for solvents, resins, and certain additives. Most folks working outside science or manufacturing never hear about it.
If you spend time reading material safety data sheets or talking to chemists, you start to learn which chemicals demand attention. Handling 2,4,5-Trimethylphenol needs care. Skin contact or inhalation can cause irritation. Shortness of breath, coughing, or burning sensations pop up in technical literature as possible symptoms. Direct eye exposure produces immediate stinging. Chronic health effects still need more research, but it’s not a compound to brush off as harmless.
Some scientific sources say it may damage nerves or disrupt organ function if a person gets substantial or repeated exposure. The Environmental Protection Agency and European Chemical Agency flag phenols, in general, for their hazardous potential. No one should assume this type of chemical blends into the environment without a trace, or that body systems shrug off its effects.
Spills and leaks attract attention fast. Trimethylphenols break down slowly in soil and water, raising red flags. Fish and water invertebrates are sensitive to phenolic compounds, and just a small amount makes an ecosystem struggle. During a site audit years ago, I walked past pipes where even a tiny drip needed an immediate cleanup protocol. The risk to wildlife and water quality can spread, especially where regulations fall short.
Experience with hazardous materials drives home the need for strong rules and responsible handling. Companies keep 2,4,5-Trimethylphenol in sealed systems, putting an emphasis on personal protective equipment—goggles, gloves, and detailed emergency procedures. Engineering controls mean fume hoods and specialized ventilation. Government inspectors do not give warnings, they issue orders when they see lapses.
Efforts in recent years focus on tighter labeling, better training for industrial workers, and record-keeping that holds companies to account for spills or improper storage. Regular audits reinforce the idea that safety depends as much on attitude as equipment; cutting corners with phenolic chemicals always comes back to haunt an operation.
Replacement with less toxic alternatives creates new opportunities. Green chemistry research teams make strides, developing substances with the same utility but fewer dangers. Some places push for environmental impact studies long before a production chain gets rolling. Policy changes in the EU and the US reflect growing public concern—nobody wants to wait for accidents and illnesses to drive reform.
Better transparency around risks and safety data helps everyone from workers to nearby communities make informed choices. Switching to closed-loop production, tightening emission controls, and boosting emergency preparedness pay off. In settings where 2,4,5-Trimethylphenol persists, ongoing research and strict oversight remain worth every penny.
Respect for chemicals like 2,4,5-Trimethylphenol starts with honest communication about health and environmental impacts. Trust builds through shared responsibility—routine training, investment in safe equipment, and real consequences for negligence. The goal isn’t just to comply with the law; it’s to make sure risks shrink and safer alternatives rise.
Workers and community members benefit when businesses treat hazard information as something to discuss openly, not to bury in technical jargon. Real progress comes from listening to those closest to the job, supporting new solutions, and always keeping a sharp eye on long-term effects. True safety grows in that space, step by step.
I’ve spent years around chemical warehouses, listening to stories about near-misses and spills that could have gone sideways. Stubborn aromas from poorly sealed containers cling to your work clothes and nose, so the importance of storing something like 2,4,5-Trimethylphenol never feels theoretical. This compound carries both fire risk and the ability to irritate skin, eyes, and your lungs. Let’s talk straight about what works to keep everyone safe.
Materials like 2,4,5-Trimethylphenol belong in a spot designed for chemicals, not the shelf by the coffee machine or a dark corner in the workshop. Steel drums or thick glass bottles with tight-fitting caps usually do the job. Even a tiny leak can fill the room with an unpleasant, sharp smell. Vapors aren’t just a nuisance — they can ignite. Locking containers in a cool, well-ventilated room limits both inhalation risk and fire danger. Nobody likes the idea of a flash fire because ventilation got ignored or a door wasn’t shut.
Some old habits in smaller shops involve reused plastic jugs or open bins. That may save a buck, but those shortcuts invite moisture or direct sunlight, both of which can start slow breakdown or spoilage in aromatic phenols. Chemical storage cabinets — marked and separated by hazard class — solve more problems than they create. Fire-resistant cabinets also buy precious time if something does go wrong.
A day’s work in a lab or plant rarely goes as planned. Gloves (nitrile or neoprene work well), chemical splash goggles, and long sleeves are never overkill. One quick slip while weighing out powder or pouring into a beaker, and you’ve got painful rashes or worse. Years ago, I watched someone try to wash their eyes under a clogged emergency station. That’s the kind of memory that sticks. Eye-wash stations, safety showers, and proper first-aid kits shouldn’t collect dust.
Chemical safety data sheets spell out accident response steps in plain language — and they don’t sit buried in a binder. Regular safety drills let everyone practice what to do after a spill or fire. Responding from memory saves time, which can be the difference between a simple cleanup and a call to the hospital.
Good practices don’t end when the work’s done. Any leftover 2,4,5-Trimethylphenol goes into sealed, labeled hazardous waste containers. Never dump this chemical down the drain or toss leftover solids in the regular trash. Disposal services exist for a reason. Environmental fines and cleanups cost far more than a scheduled waste pickup.
The American Chemical Society and OSHA both offer free, up-to-date information on storage and handling for chemicals like this. I’ve learned that the most reliable advice comes from industry standards, not a random forum or an outdated textbook. Updates in safety guidelines don’t happen to make life difficult — they come from hard-earned lessons, sometimes from real accidents.
Respecting chemicals like 2,4,5-Trimethylphenol starts with understanding how they fit in a workplace routine and ends with sending everyone home without stories to tell about a bad day at the warehouse. Regular training, up-to-date labels, and the right gear keep risk where it belongs: under control.
2,4,5-Trimethylphenol has the molecular formula C9H12O. If we break that down, we're looking at a benzene ring bonded to three methyl groups and a single hydroxyl group. On paper, the numbers tell you it features nine carbons, twelve hydrogens, and one oxygen. That oxygen comes from a single hydroxyl group (-OH), making it a type of phenol—basically a hydroxybenzene with extra methyls attached.
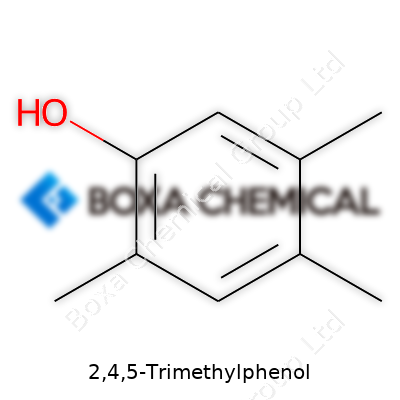
For 2,4,5-Trimethylphenol, the methyl groups land at the 2, 4, and 5 positions on the benzene ring. The hydroxyl group sits right at the “1” spot, really anchoring the molecule as a phenol. Think of the ring as a clock face, with twelve o’clock as the starting point—set the -OH at the top, then move clockwise to count off positions. Methyls go onto carbons two, four, and five. This arrangement shapes much of how the molecule behaves, especially in chemical reactions or industrial processes.
Substitution on the benzene ring does more than change the shape of the molecule—it shifts the way those atoms interact. People working in chemistry labs know that, depending on where methyl and hydroxyl groups sit, you might see big swings in reactivity, solubility, maybe even toxicity. I remember years ago during a synthesis project, swapping the placement of a methyl group made a night-and-day difference in how the compound reacted with oxidizing agents. Minor tweaks often create brand-new properties.
The positioning of the methyl groups on a ring system like this can influence how easily the molecule mixes or separates from other compounds. In environmental chemistry, that means it may stick around longer or end up moving through water or soil in unexpected ways. In manufacturing, a certain position can affect the smell, flavor, or performance of a phenol-based resin or antioxidant. Chemists pay attention to that; getting the orientation wrong leads to a failed batch.
2,4,5-Trimethylphenol finds its place in more than academic textbooks. This phenol derivative comes into play in industries making antioxidants, fragrances, and even some resins. The methyl groups can boost stability, which extends a product’s shelf life or may keep it from breaking down under harsh conditions. For anyone involved in making paints or plastics, a stable molecule helps the final product stand up to heat and exposure. I’ve seen companies use variants of this molecule to tweak the scent of household cleaners, taking a basic chemical backbone and spinning off something market-ready.
With most phenolic compounds, safety pops up as a concern. Benzene derivatives sometimes bring environmental questions, and 2,4,5-Trimethylphenol fits that narrative. In the lab, it’s smart to work with proper ventilation and gloves. On the production floor, storage conditions call for close monitoring to avoid oxidation or unwanted reactions.
Some folks push for safer, bio-based phenol alternatives to cut down on environmental risks. Investment in green chemistry offers hope, because tailored molecules may eventually replace those causing safety headaches. I’ve watched the shift to more sustainable input chemicals for resins and coatings, as regulations tighten up and companies look for friendlier options. Better labeling, better data, and real-world studies make a difference for users, workers, and communities living next to production sites.
More transparent reporting and improved analytical methods bring confidence to both industry and consumers. Open data on environmental effects helps everyone weigh trade-offs between usefulness and potential harm. Advances in synthetic chemistry mean it’s possible to nudge this basic chemical scaffold in safer, greener directions. Mixing practical knowledge with safer science keeps both performance and people’s well-being at the forefront.
| Names | |
| Preferred IUPAC name | 2,4,5-Trimethylphenol |
| Other names |
2,4,5-Trimethylphenol Phenol, 2,4,5-trimethyl- Tmesol |
| Pronunciation | /tuː, fɔːr, faɪv traɪˈmɛθɪlˌfiːnɒl/ |
| Identifiers | |
| CAS Number | 89-72-5 |
| Beilstein Reference | 1209226 |
| ChEBI | CHEBI:34464 |
| ChEMBL | CHEMBL206358 |
| ChemSpider | 136112 |
| DrugBank | DB02523 |
| ECHA InfoCard | 03e80ead-7775-47b3-ae3a-3d4190ab9ee0 |
| EC Number | 205-611-3 |
| Gmelin Reference | 94359 |
| KEGG | C01789 |
| MeSH | D018325 |
| PubChem CID | 6979 |
| RTECS number | GO8575000 |
| UNII | Y37E3J86RN |
| UN number | UN2324 |
| CompTox Dashboard (EPA) | DTXSID6020286 |
| Properties | |
| Chemical formula | C9H12O |
| Molar mass | 150.22 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Phenolic odor |
| Density | 0.994 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 2.81 |
| Vapor pressure | 0.049 hPa (25 °C) |
| Acidity (pKa) | 10.84 |
| Basicity (pKb) | 8.58 |
| Magnetic susceptibility (χ) | -59.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Viscosity | 3.22 mPa·s (25 °C) |
| Dipole moment | 1.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 188.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -187.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4557.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2,4,5-Trimethylphenol: 2-2-0 |
| Flash point | Flash point: 110 °C |
| Autoignition temperature | 425 °C |
| Explosive limits | Explosive limits: 1.1–6.4% |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 820 mg/kg (rat, oral) |
| NIOSH | SE3850000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of 2,4,5-Trimethylphenol: Not established |
| REL (Recommended) | REL: 2 ppm (10 mg/m³) |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
2,4,6-Trimethylphenol 2,3,5-Trimethylphenol 2,3,6-Trimethylphenol 2,3,4-Trimethylphenol 3,4,5-Trimethylphenol |